Water Solubility, Polarity, and Hydrophobicity in Drug Chemistry
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
Why must nearly every drug be water soluble?
Because all of chemistry, including physiological chemistry, occurs in solution.
What is the significance of polarity in relation to water solubility?
determines chemical characteristics of a substance that affect its ability to dissolve in water.
What does electronegativity measure?
how strongly atoms attract bonding electrons to themselves.
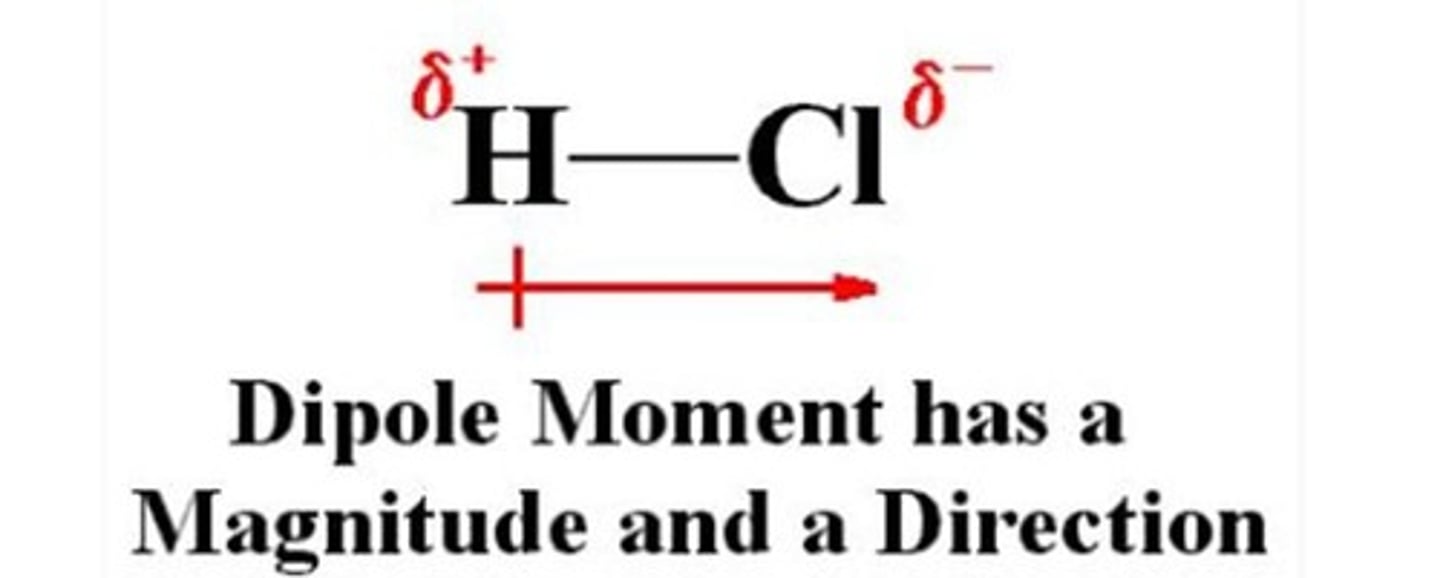
What indicates a polar bond?
a dipole moment due to unequal sharing of electrons between different atoms.
Which atoms typically create polar bonds when bonded to non-metals?
Fluorine (F), Nitrogen (N), Oxygen (O), and Chlorine (Cl).
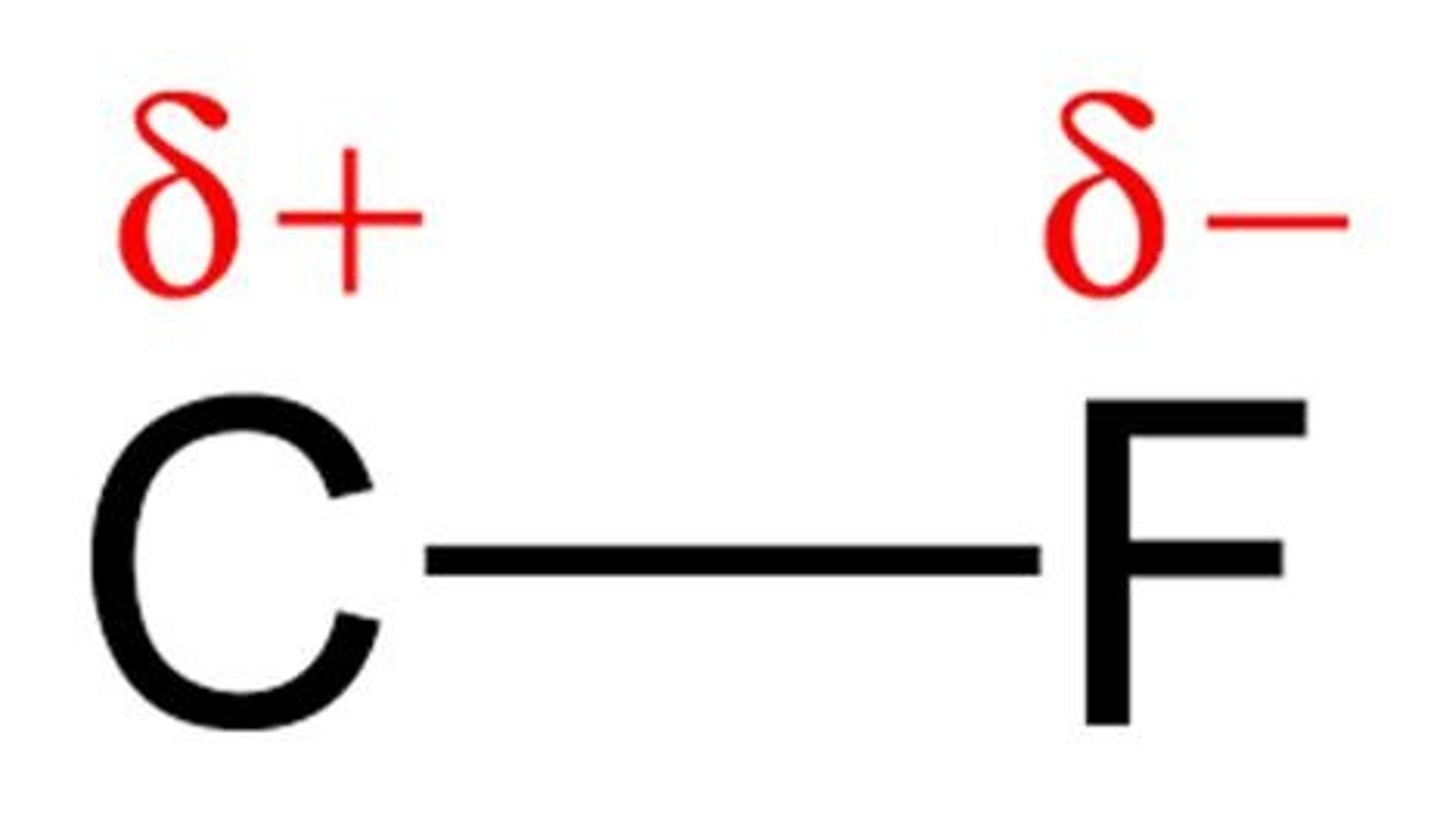
What symbols are used to indicate electron density in polar bonds?
ẟ- (atom with greater electron density), & ẟ+ (atom with lesser electron density)
What role do hydrogen atoms attached to nitrogen or oxygen play in water?
serve as hydrogen bond donors.
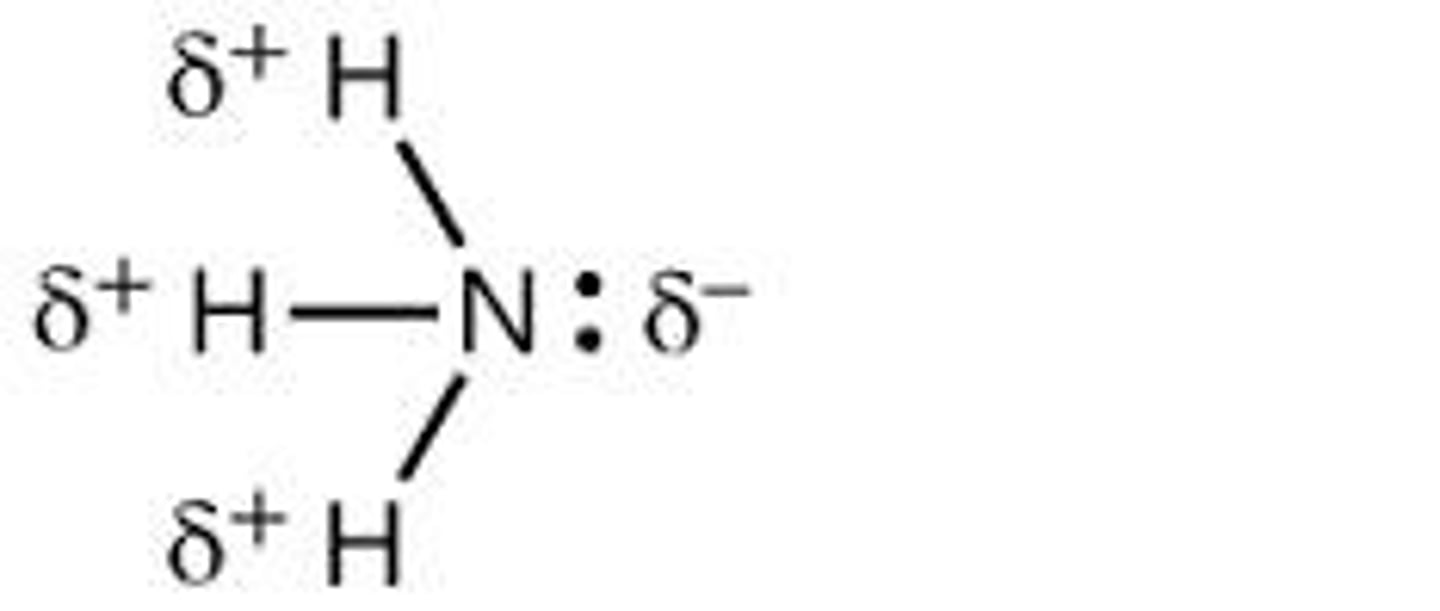
What characterizes a non-polar covalent bond?
Equal or nearly equal sharing of e- between two atoms, with e- difference of 0.5 or less.
What is a polar covalent bond?
A bond with unequal sharing of electrons, typically with an electronegativity difference between approximately 0.5 and 1.9.
What defines an ionic bond?
complete transfer of electrons between two atoms, generally occurring when the electronegativity difference is greater than 2.0.
What are examples of substances with ionic bonds?
Sodium chloride (NaCl) and magnesium chloride (MgCl2).
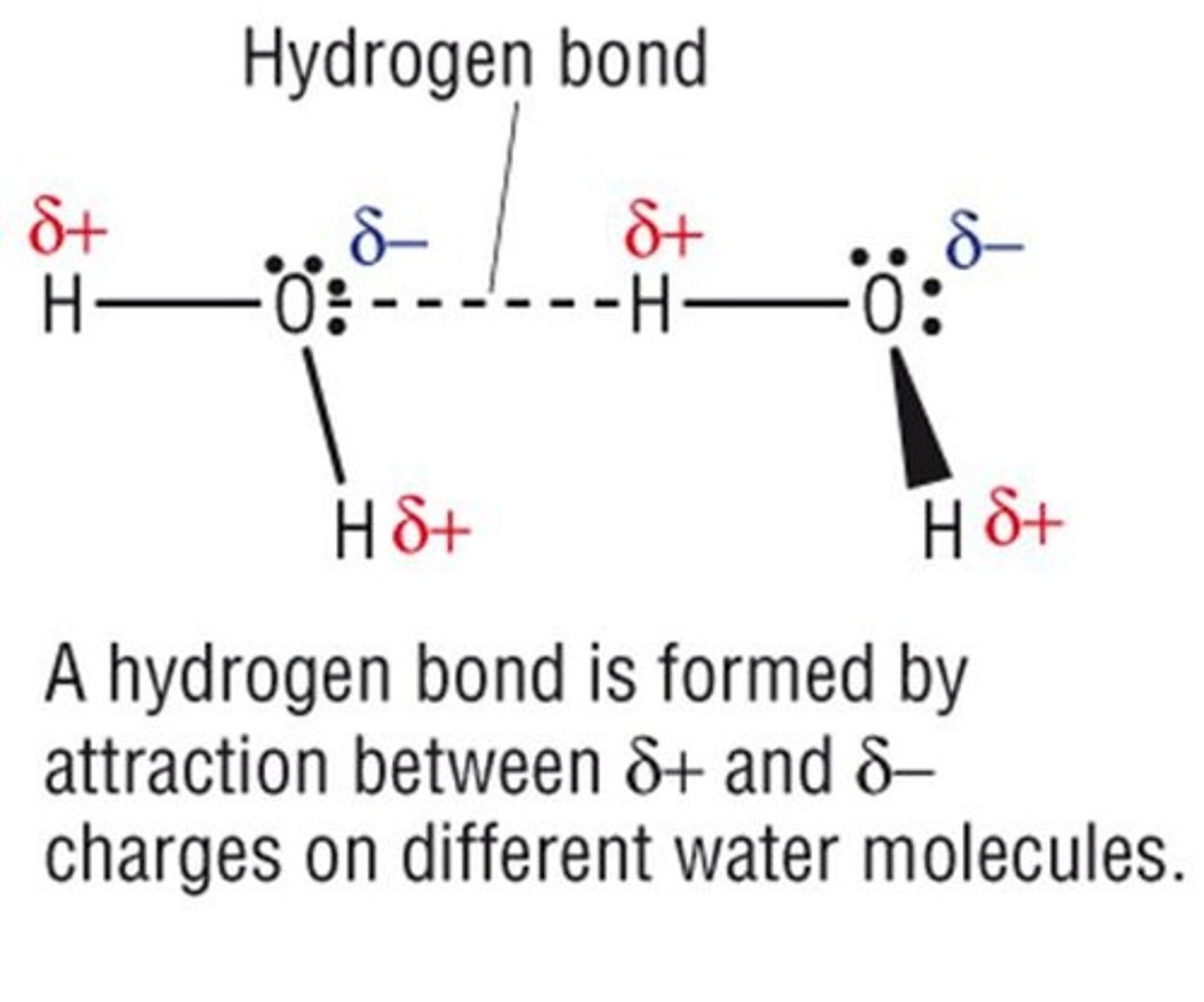
What is a hydrogen bond?
An electrostatic force of attraction between a hydrogen atom covalently bound to a more electronegative atom and another electronegative atom with a lone pair of electrons.
Which atoms are common hydrogen bond donors in drug chemistry?
Hydrogen atoms attached to nitrogen (N-H) or oxygen (O-H) atoms.
What types of atoms can serve as hydrogen bond acceptors?
Atoms with a lone pair of electrons, specifically nitrogen, oxygen, or fluorine.
Can halogen atoms participate as hydrogen bond acceptors?
No
Can negatively charged oxygen or nitrogen atoms participate in hydrogen bonding?
Yes
What is the electronegativity difference range for polar covalent bonds?
Approximately 0.5 (barely polar) to 1.9 (about as polar as a bond can get without being ionic).
What is the maximum electronegativity difference for a bond to be considered non-polar covalent?
0.5 or less.
What is the typical upper limit for a bond to be classified as polar covalent?
Some textbooks list 1.7 as the upper limit.
What is the significance of the dipole moment in a bond?
It indicates that the electrons are not equally shared due to differences in electronegativity.
What is the role of lone pairs in hydrogen bonding?
Lone pairs on electronegative atoms can accept hydrogen bonds.
What is the relationship between electronegativity and bond polarity?
Greater differences in electronegativity between bonded atoms lead to more polar bonds.
What is the convention for designating electron density in polar bonds?
Use ẟ- for the atom with greater electron density and ẟ+ for the atom with lesser electron density.
Why can't carbon atoms attached to hydrogen function as hydrogen bond donors?
Because the C-H bond does not exhibit significant polarity or dipole moment, as the electrons are shared more equally.
What type of solvent is water and what does it exhibit?
Water is a polar solvent with a dipole moment.
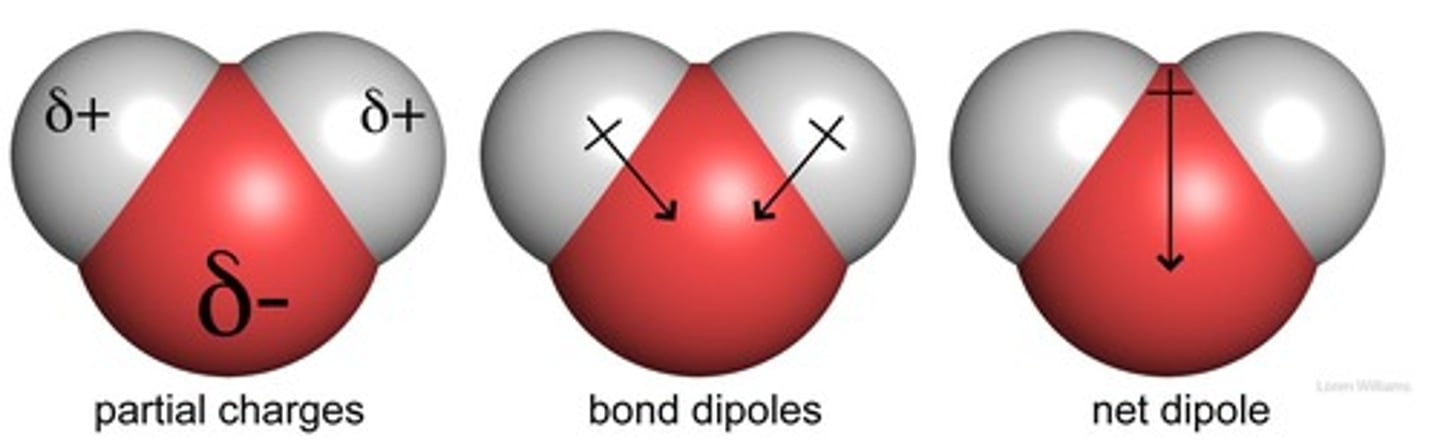
How does the oxygen atom in a water molecule interact with hydrogen atoms?
The oxygen atom attracts a greater share of the electrons in each bond it shares with hydrogen.
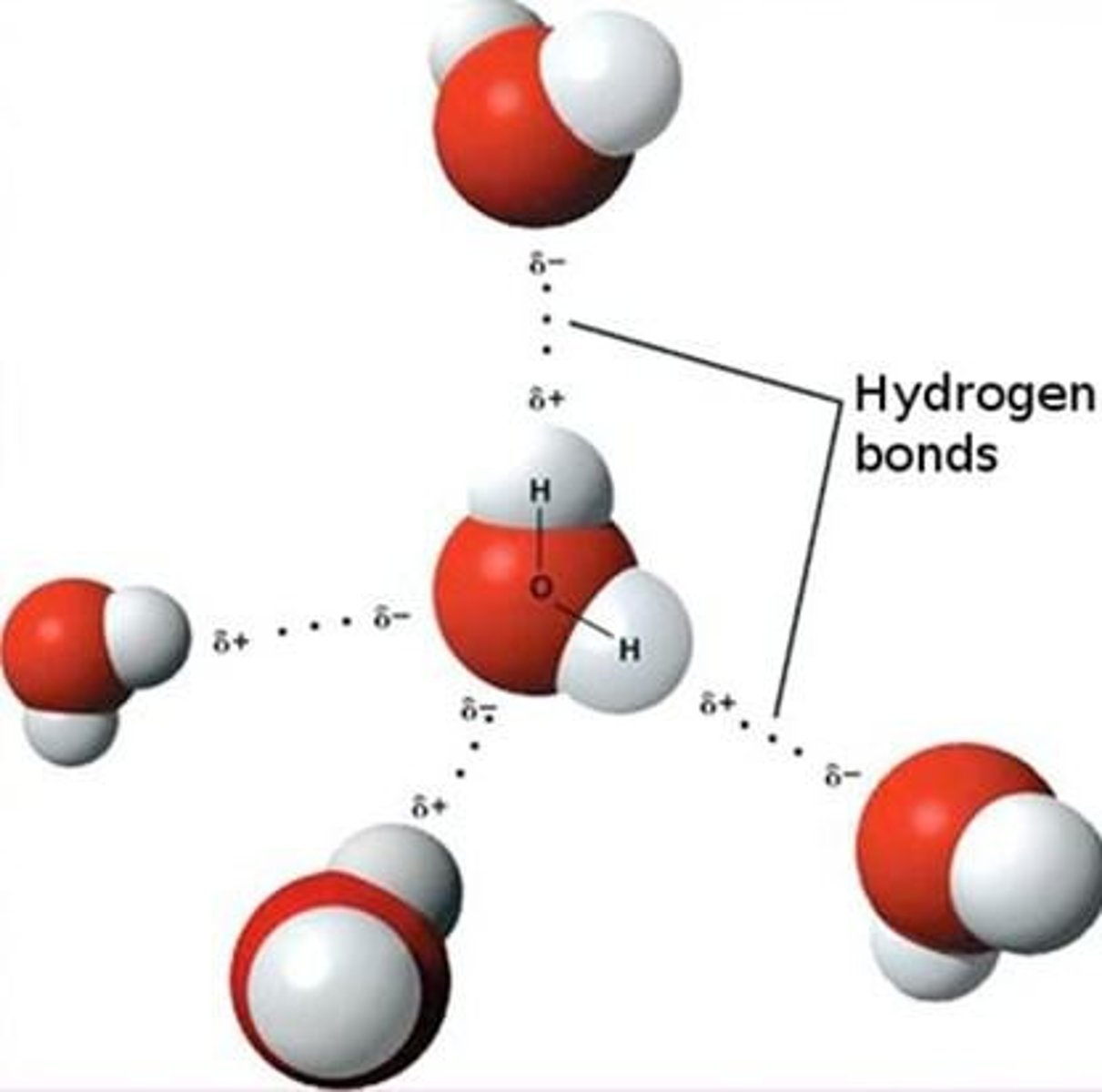
What role do lone pairs of electrons on the oxygen atom of water play?
They serve as significant sources of electron density and can act as hydrogen-bond acceptors.
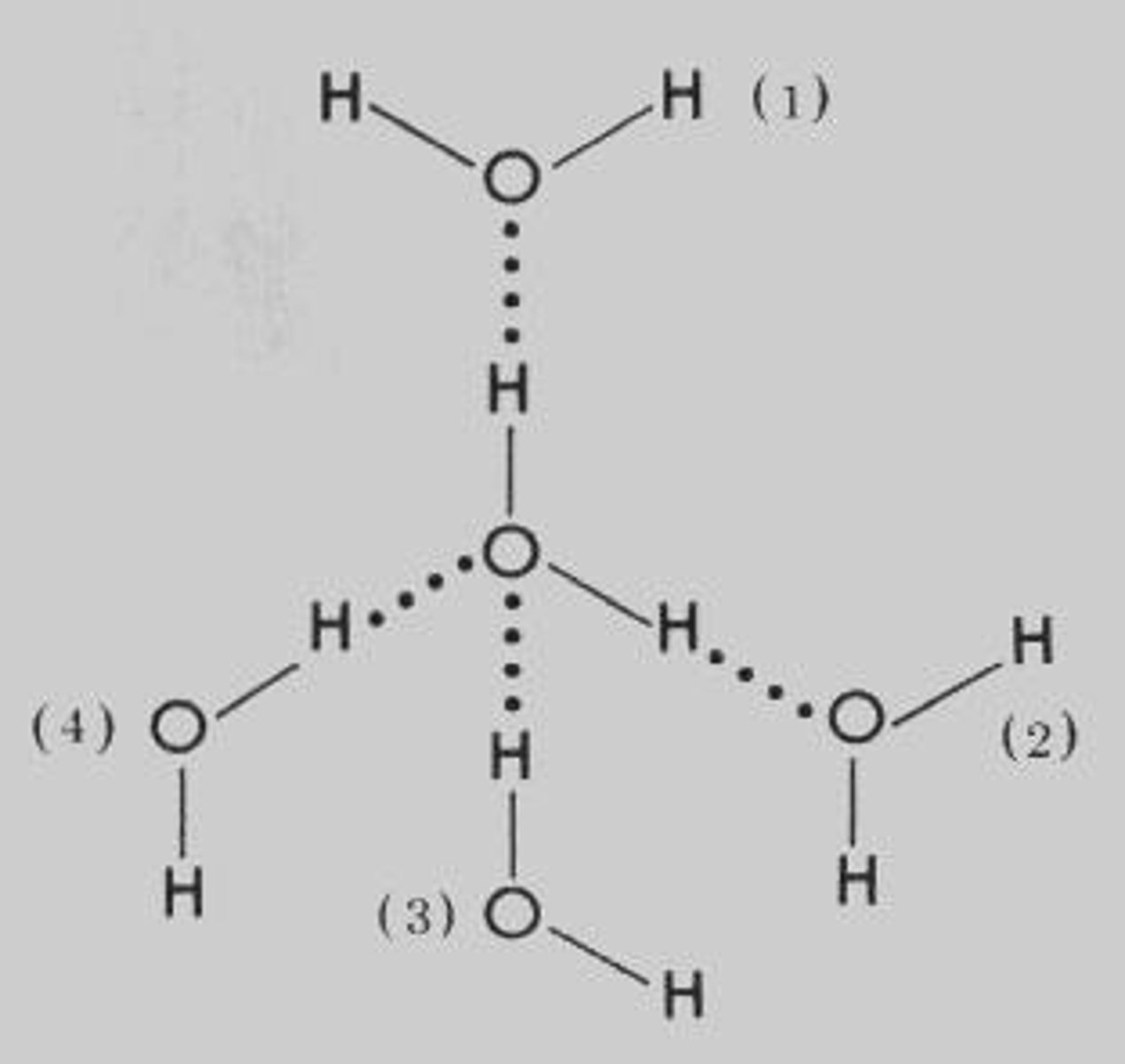
How many hydrogen bonds can a single water molecule form?
An individual water molecule can form four hydrogen bonds.
What is meant by 'flickering clusters' in the context of water?
In bulk water, hydrogen bonds continually form and break with other water molecules, averaging 3.4 hydrogen bonds per molecule.
What are the two types of compounds that are generally water soluble?
(1) Ionized compounds with full positive or negative charges, and (2) compounds that can hydrogen bond with water.
What term is used to describe compounds that are water soluble?
Hydrophilic.
What characterizes lipid-soluble substances?
They are non-polar and do not contain covalent bonds with significant polarity, making them water-insoluble.
What are examples of lipid-soluble compounds?
Hydrocarbons and large halides (Cl, Br, I) covalently bonded to organic molecules.
What is the term used for compounds that are lipid soluble?
Lipophilic (or hydrophobic).
How does water dissolve salts?
Water forms a shell of interacting molecules around individual ions, weakening electrostatic interactions and counteracting crystal lattice formation.
What happens to the entropy when a salt dissolves in water?
There is a large increase in entropy as the individual ions become more mobile.
What is the thermodynamic favorability of salt dissolution in water?
The change in free energy is very negative, making dissolution thermodynamically favored.
What does it mean for a drug to be amphipathic?
It contains both hydrophilic and lipophilic (hydrophobic) portions.
What is an example of an amphipathic molecule in cell membranes?
Phospholipids, which have polar heads and fatty non-polar tails.
What is the difference between amphipathic and amphoteric?
Amphipathic refers to molecules with both hydrophilic and lipophilic parts, while amphoteric refers to substances that can act as both acids and bases.
What percentage of orally-administered drugs are absorbed into the bloodstream?
95%.
What process allows drugs to be absorbed through small intestine mucosal cells?
Passive diffusion through the lipid bilayer of the mucosal cell's plasma membrane.
What is the significance of the polarity of amino acid side chains?
It affects the solubility and interactions of amino acids in biological systems.
What is the partition coefficient?
It is a measure of how a compound distributes between two immiscible phases, often used to predict drug absorption.
Can you identify the hydrophilic and lipophilic portions of the amphipathic drug Fenofibrate?
The hydrophilic portion is the polar head, while the lipophilic portion is the fatty tail.
What type of drugs can more readily cross lipid bilayers?
Lipophilic drugs
What is the relationship between hydrophilic compounds and water solubility?
Hydrophilic (polar and/or charged) compounds are more water soluble than non-polar ones.
What must an oral drug balance to be effective?
An oral drug must balance being lipophilic enough to cross a membrane bilayer for absorption and hydrophilic enough to be water soluble.
What type of compounds are well-suited to be drugs?
Amphipathic compounds.
What does the partition coefficient measure?
The differential ability of a solute to dissolve between two immiscible phases.
What are the two phases typically involved in measuring the partition coefficient?
One phase is non-polar (often octanol), and the other phase is always water.
How is the oil-to-water partition coefficient calculated?
It is calculated as the logarithm of the ratio of the concentration of the drug in the nonpolar phase to the concentration of the drug in water.
What is the formula for the partition coefficient (PC) when octanol is the non-polar phase?
PC = [concentration of drug in octanol] / [concentration of drug in water].
What is the common term used to describe the partition coefficient?
Log P.
What is the Fragment Method in calculating partition coefficients?
It involves assigning a pi (π) value to each fragment of the molecule and summing these values to predict the partition coefficient.
What is the π value for an aliphatic carbon (C)?
+ 0.5.
What is the π value for a phenyl group (C6H5)?
+ 2.0.
What is the enthalpy of solution?
The enthalpy change (ΔH) associated with the dissolution of a substance in a solvent.
How is the enthalpy of solution typically expressed?
In kJ/mol at constant temperature.
What are the three steps of dissolution?
1. Breaking solute-solute attractions (endothermic), 2. Breaking solvent-solvent attractions (endothermic), 3. Forming solvent-solute attractions (exothermic).
What is the dominant intermolecular force in liquid water?
Hydrogen bonding.
Why do lipids and water not mix appreciably?
Lipids are nonpolar and cannot hydrogen bond, while water molecules are held together by strong hydrogen bonds.
What forces hold lipid molecules together?
Dispersion (van der Waals) forces.
What must be overcome to form a lipid-water solution?
The strong water-to-water hydrogen bonding and the dispersion forces between lipid molecules.
What is the significance of the energy released by solvation in lipid-water interactions?
Very little energy is released, making ΔH small and insignificant.
What is the role of hydrogen bonding in the mixing of lipids and water?
Hydrogen bonding is the dominant force in water, preventing mixing with nonpolar lipids.
What happens to the energy change during the dissolution process?
It consists of endothermic and exothermic components: breaking bonds requires energy, while forming new attractions releases energy.
What is the driving force for the separation of water and hydrocarbons?
The hydrophobic effect, which is an entropy-driven process.
What is the relationship between ΔG, ΔH, and ΔS in spontaneous processes?
For a process to be spontaneous, ΔG must be negative, where ΔG = ΔH - TΔS.
What happens to water molecules when a non-polar solute is placed in water?
Water molecules tend to reorient around the non-polar molecule, forming hydrogen-bonded 'cages'.
Why do water molecules in cages have less entropy?
They are more highly organized and restricted in their interactions compared to bulk water.
How much entropy do individual water molecules in cages lose?
Each water molecule in the cage loses up to 2 entropy units (approximately 0.6 kcal/mole).
What occurs when non-polar molecules aggregate in water?
The number of ordered water molecules decreases, leading to an increase in entropy.
What is the energetically favorable outcome of non-polar molecules clustering together?
The release of ordered water molecules increases the randomness (entropy) of the system.
What is the hydrophobic effect?
The tendency of non-polar molecules to cluster together in water to minimize surface area exposure, increasing entropy.
How does the hydrophobic effect influence protein folding?
It drives globular proteins to bury their hydrophobic residues in the interior and expose polar residues to the surface.
What is the stabilization energy associated with burying a -CH2 group in a protein?
Approximately 0.8 kcal stabilization for each -CH2 buried.
How do phospholipids arrange themselves in a biological membrane?
The non-polar tails face inward while the hydrophilic heads face outward, in contact with water.
What role does the hydrophobic effect play in drug-receptor interactions?
It facilitates the binding of non-polar portions of drugs to hydrophobic pockets on receptor proteins.
What happens to the total surface area exposed to water when hydrophobic molecules aggregate?
The total surface area exposed to water decreases.
Why is the aggregation of non-polar molecules energetically favorable?
It results in more 'free' water molecules, increasing the entropy of the system.
What type of structures do water molecules form around hydrocarbons?
Cage-like structures of highly ordered water molecules.
What is the significance of the hydrophobic effect in cellular membranes?
It is crucial for the formation of lipid bilayers in biological membranes.
What type of interactions occur between non-polar drug portions and receptor sites?
Non-polar drug portions interact with hydrophobic pockets on the receptor site.
How does the hydrophobic effect contribute to the stability of proteins?
By driving the burial of hydrophobic residues, which stabilizes the protein structure.
What is the impact of water molecule organization on the entropy of the system?
More organized water molecules lead to decreased entropy, while less organized (free) water increases entropy.
What is the effect of non-polar solute dilution on water molecule organization?
Highly diluted non-polar solutes lead to more organized water cages around them.
What is the significance of the term 'cage' in the context of the hydrophobic effect?
It refers to the ordered arrangement of water molecules around non-polar solutes.
What happens to the entropy of water molecules when they are released from cages?
Their entropy increases, contributing to the overall increase in system entropy.
How does the hydrophobic effect relate to the concept of randomness in a system?
The aggregation of non-polar molecules increases randomness by freeing water molecules from ordered states.