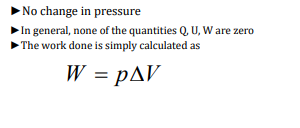FIRST LAW OF THERMODYNAMICS | 10.3
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
thermal equilibrium.
A macroscopic state of an isolated system can be specified only if the system is in*** ******
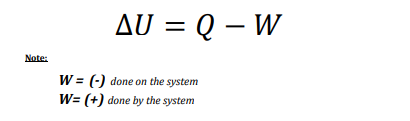
The change in internal energy of a system is the difference between the heat taken in by the system and the work done by the system.
positive (+)
heat if the energy is transferred to the system
positive (+)
work if done by the system
positive (+)
Internal energy if temperature increases
increases
More heat is added to system than system does work: Internal energy of system ***
decreases
More heat flows out of system than work is done: Internal energy of system ****.
unchanged
Heat added to system equals work done by system: Internal energy of system *****.
Adiabatic Proces

Isothermal Process
The cylinder and gas are in thermal contact with a large source of energy. ►Allow the energy to transfer into the gas (by heat). ►The gas expands and pressure falls to maintain a constant temperature. ►The work done is the negative of the heat added.

3. Isochoric Process

Isobaric Process