Dative covalent and coordinate covalent
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Strongest attractive force between two ammonia molecules
Hydrogen bonds
Draw a diagram to show how two ammonia molecules interact with each other in the liquid phase
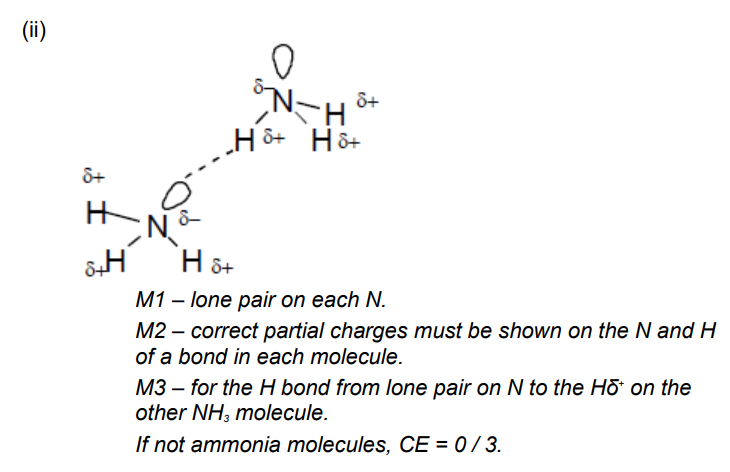
State how the bond between the ammonia and boron trichloride is formed
Lone pairs on NH3 is donated to B(Cl3)
Explain why iodine has a higher melting point than fluorine
Iodine has more electrons
stronger/ more vDW forces in between molecules
Draw shape of NHF2
Trigonal pyramid
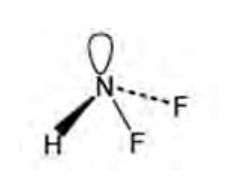
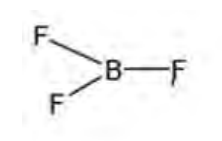
Draw shape of BF3
Trigonal planar
Suggest a value for the F-N-F bond angle in NHF2
107
How dative covalent bond is formed?
Lone pair on N(HF2) donated to BF3
State how two carbon atoms form a carbon-carbon bond in graphene
shared pair of electrons from each C atom
empirical formula of graphene
CH
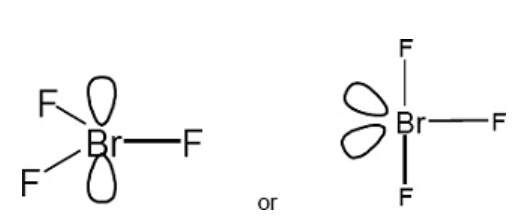
Draw the shape of BrF3 and predict its bond angle
Trigonal planar
120
or if T shaped shown its 90
Fluorine reacts with bromine to form liquid bromine trifluoride (BrF3)
state the type of bond between Br and F in BrF3,
state how this bond is formed
Covalent
shared pair of electrons from Br and one electron from F
Draw the shape of BrF4- and predict its bond angle
90
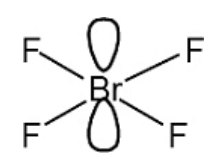
BrF4- ions are also formed when potassium fluoride dissolves in liquid BrF3 to form KBrF4
explain in terms of bonding, why KBrF4 has a high melting point
Ionic forces of attraction between ions
strong electrostatic attractions
lots of energy needed to break bonds
between K+ and BrF4- ions
Draw a diagram to show how two molecules of hydrogen fluoride are attracted to each other by the type of intermolecular forces
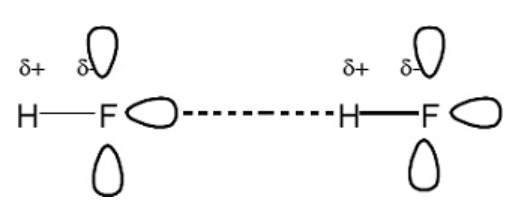
Explain why the boiling point of fluorine is very low
simple molecules
weak vDW forces in between molecules
little energy needed to overcome
Draw the shape of the PH3 molecule
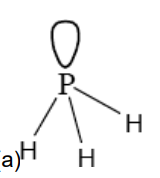
State the type of bond formed between the PH3 molecule and the H+ ion
dative covalent bond
pair of electrons on P(H3) donated to H+
Bond angle in the PH4+ ion
109.5
Although phosphine molecules contain hydrogen atoms, there is no hydrogen bonding between phosphine molecules
difference in electronegativity between P and H is too small
Write an equation for the formation of aluminium chloride from its elements
Al + 1.5 Cl2 —> AlCl3
Name the type of bond and explain how this type of bond is formed in the AlCl4- ion
dative covalent bond
electron pair on Cl- donated to Al(Cl3)
Aluminium chloride has a relative molecular mass of 267 in the gas phase
deduce the formula of the aluminium compound that has a relative molecular mass of 267
Al2Cl2
AlBr3
Deduce the name or formula of a compound that has the same number of atoms the same number of electrons and the same shape as the AlCl4- ion
SiCl4
silicon tetrachloride
Shape of TlBr5 2- ion
trigonal bipyramidal
Draw shape of the TlCl2+ ion
Cl- Tl - Cl
linear
Explain why the TlCl2+ ion has the shape (linear)
2 B.P of electrons repel equally
electrons in the bonds repel to be as far apart as possible