CHM 104 Unit 2 Study Guide
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
35 Terms
element that gains electrons as it ionizes
non-metal
element that loses electrons as it ionizes
metal
single covalent bond
two atoms held together by sharing one pair of electrons
double covalent bond
a bond in which two atoms share two pairs of electrons
triple covalent bond
a covalent bond in which two atoms share three pairs of electrons
non-polar covalent bond
a covalent bond in which the bonding electrons are shared equally by the bonded atoms, resulting in a balanced distribution of electrical charge
polar covalent bond
a covalent bond in which the bonding electrons are shared unequally by the bonded atoms, resulting in an unbalanced distribution of electrical charge
Which shape bonds can be a polar molecule?
Asymmetrical, Pyramidal, Bent
cycloalkane
cyclic hydrocarbon that contains only single bonds
methane
CH4
ethane
C2H6
propane
C3H8
butane
C4H10
pentane
C5H12
hexane
C6H14
heptane
C7H16
hydrocarbon
a compound made up of only hydrogen and carbon atoms
cyclopropane
C3H6
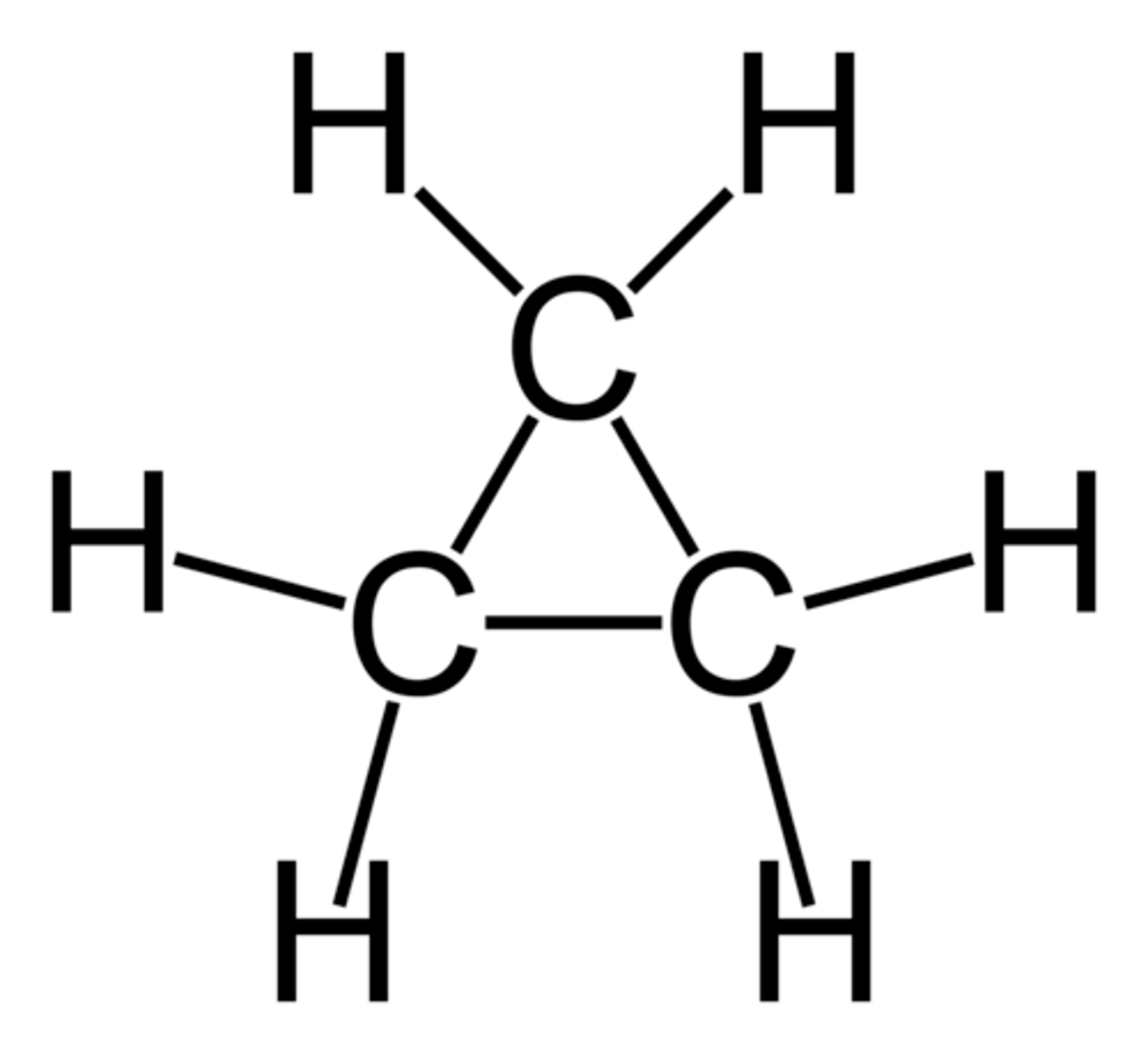
cyclobutane
C4H8
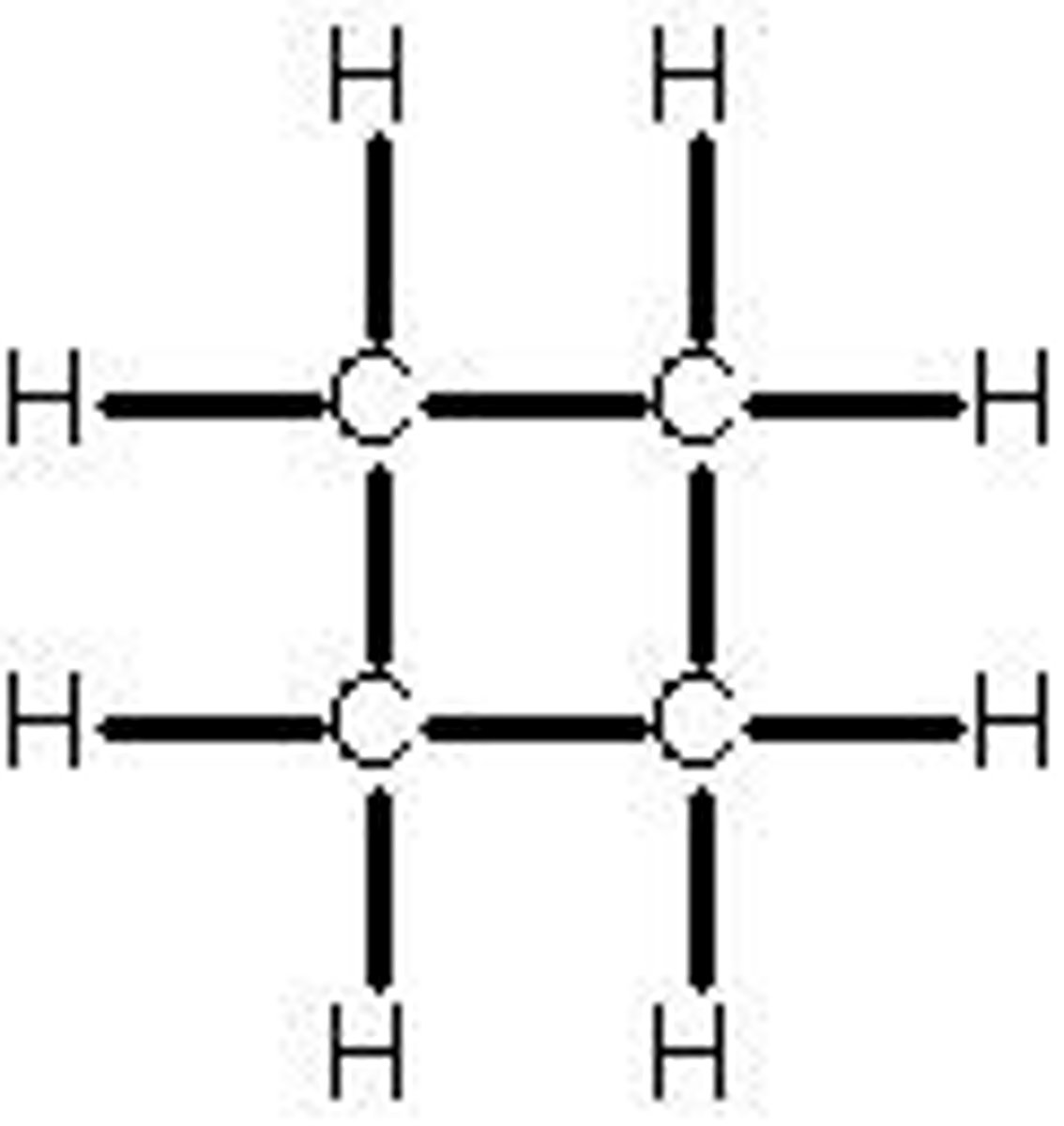
cyclopentane
C5H10
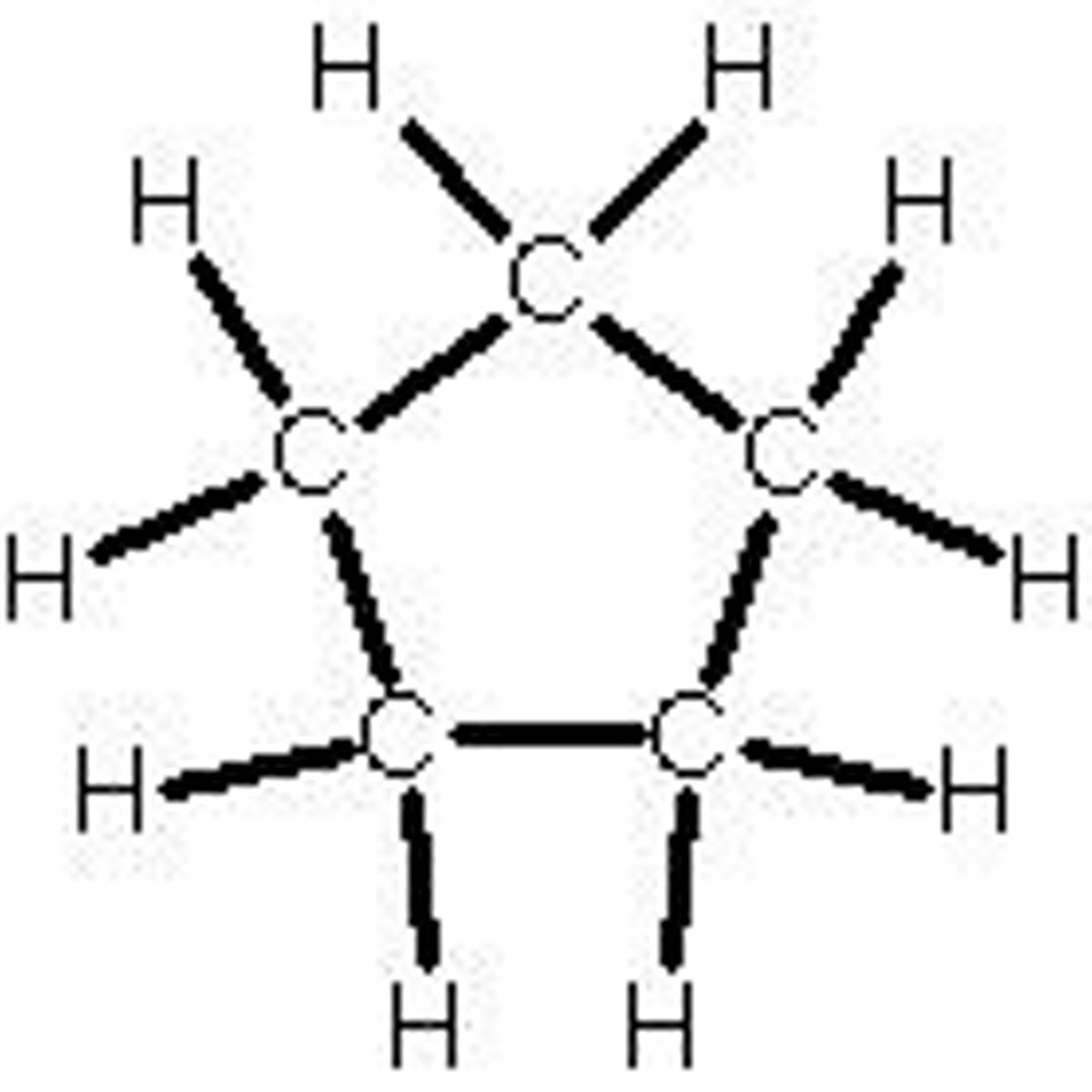
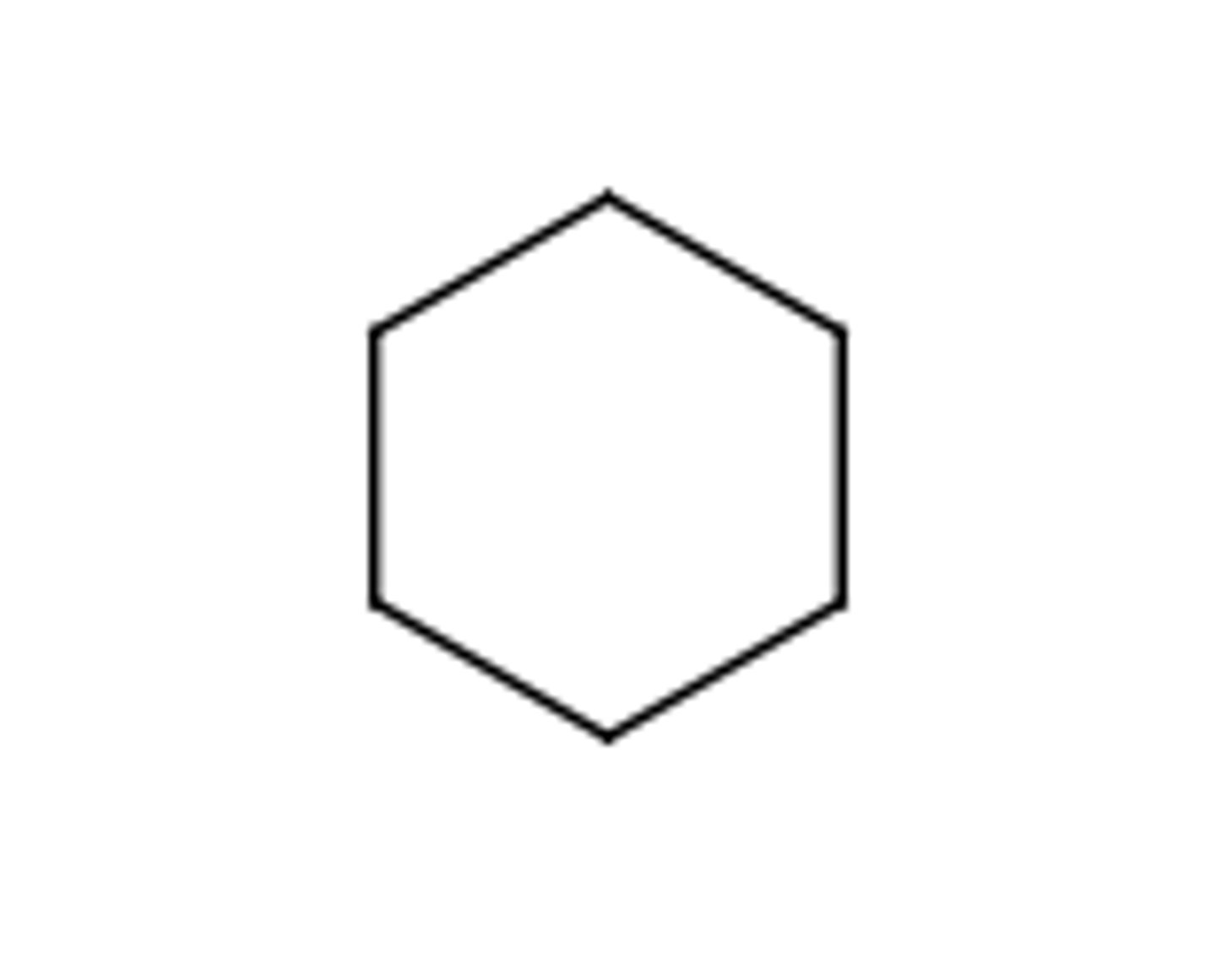
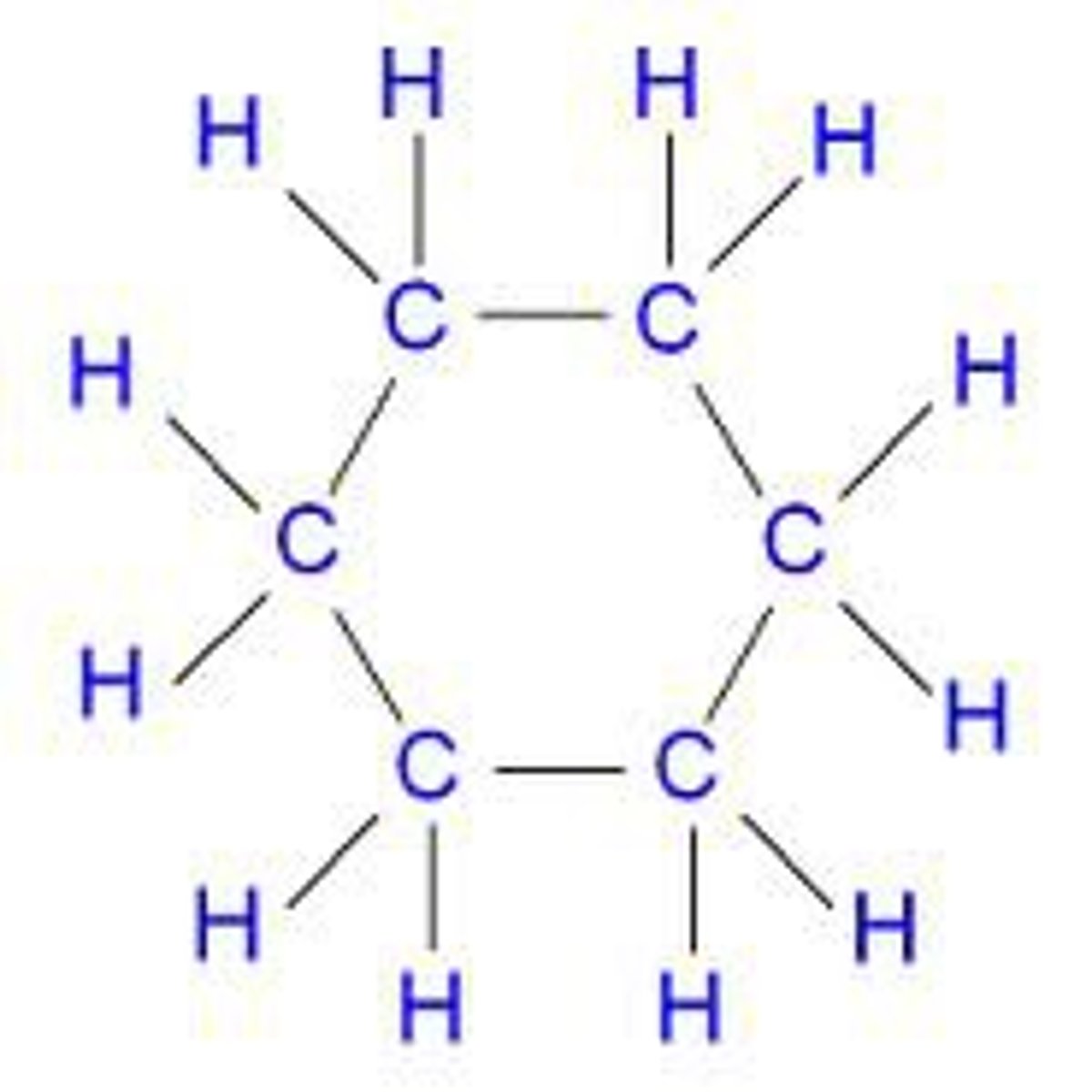
cyclohexane
C6H12
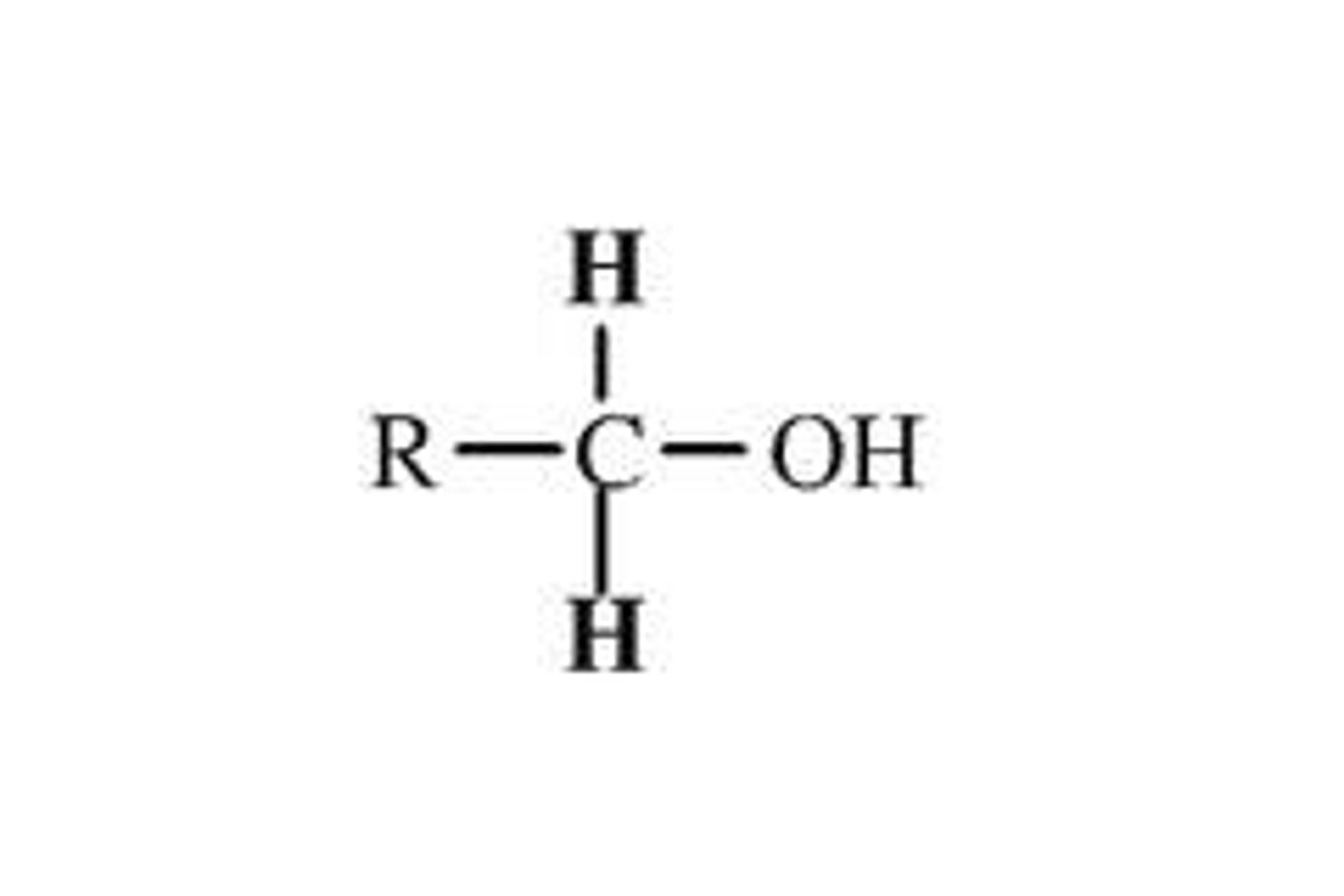
primary alcohol
the -OH of alcohol is attached to an end carbon
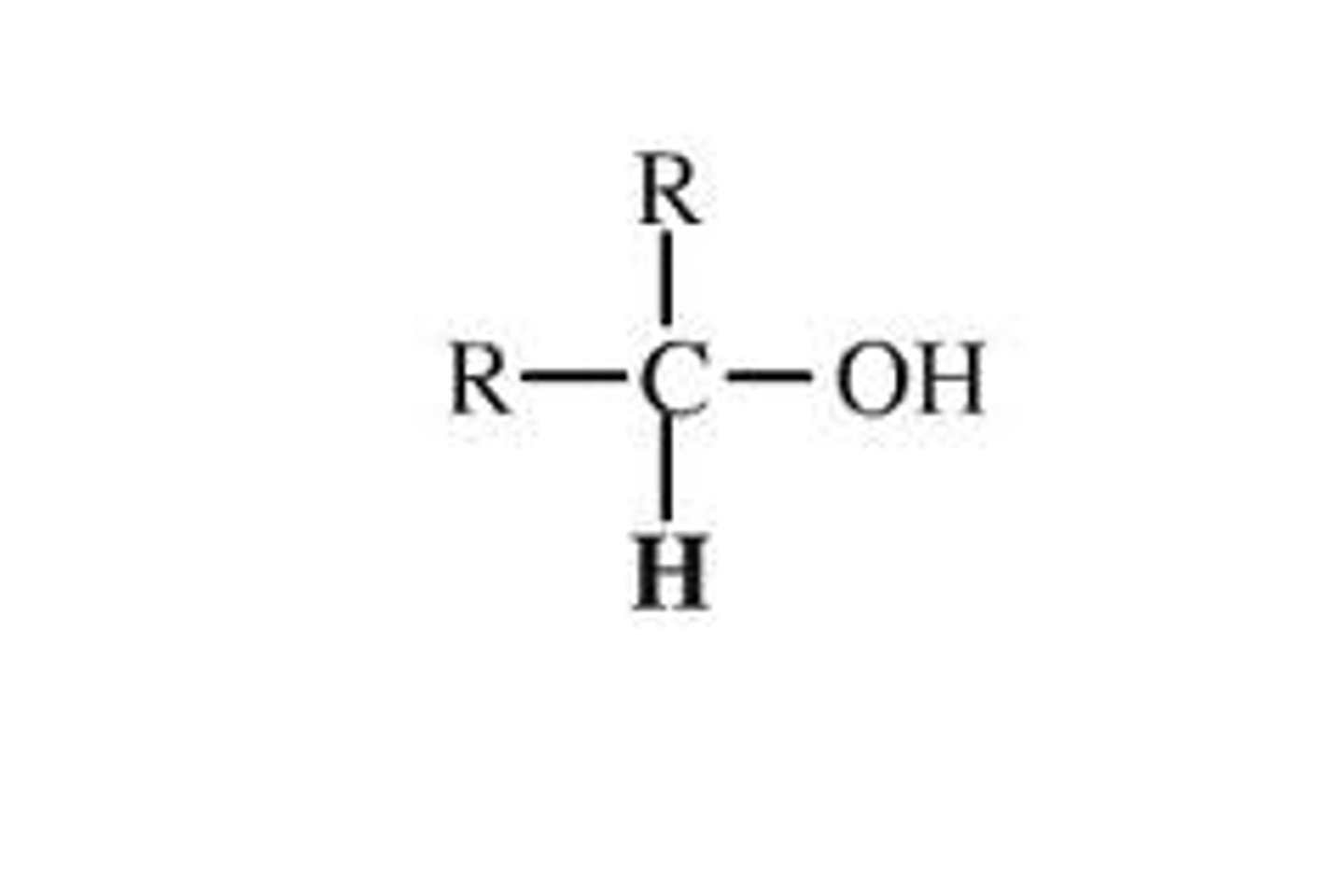
secondary alcohol
the -OH group is attached to a carbon atom that is attached to two carbon chains
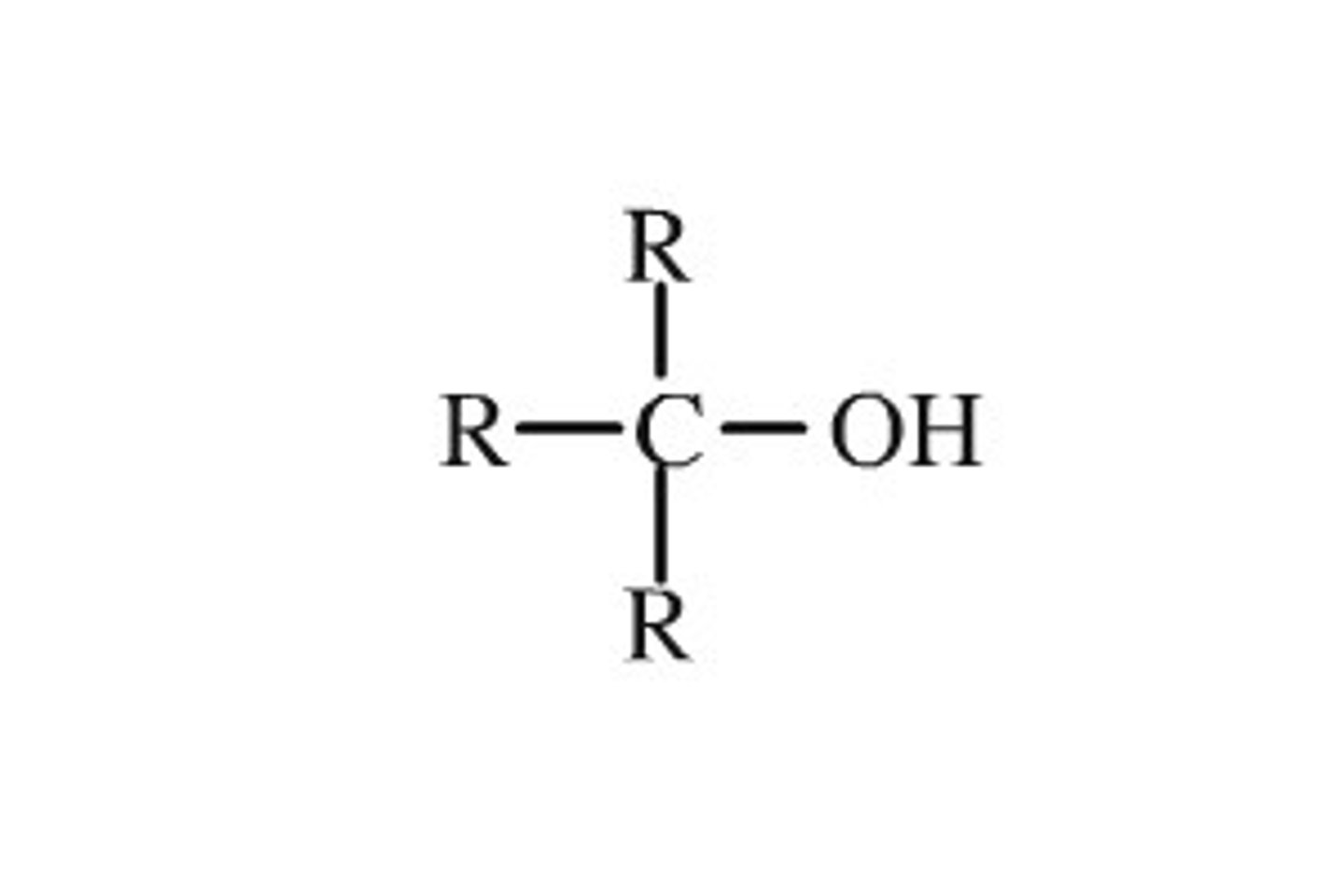
tertiary alcohol
the -OH group is attached to a carbon atom that is attached to three carbon chains
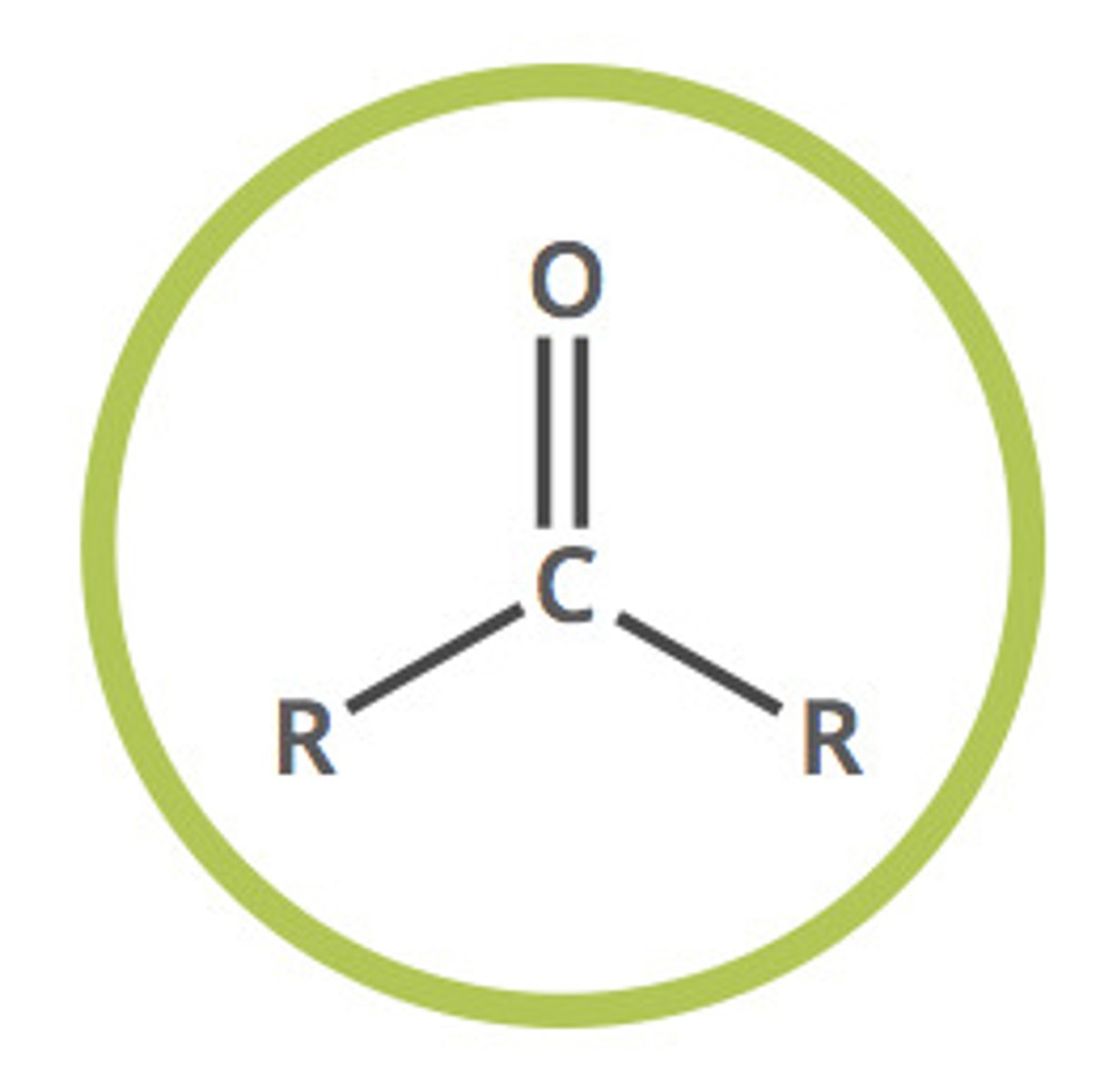
ketone
R-C=O-R
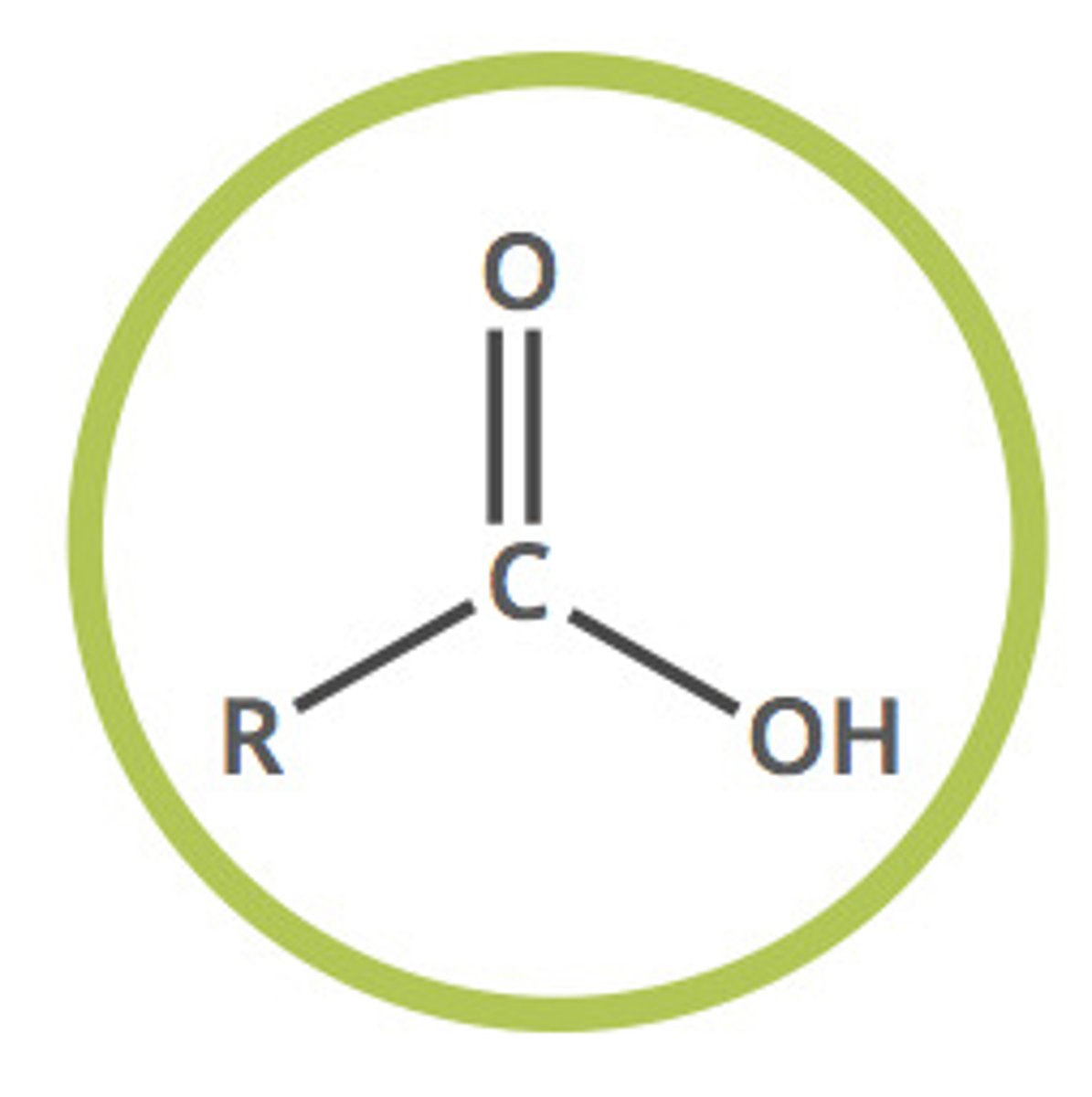
carboxylic acid
R-COOH
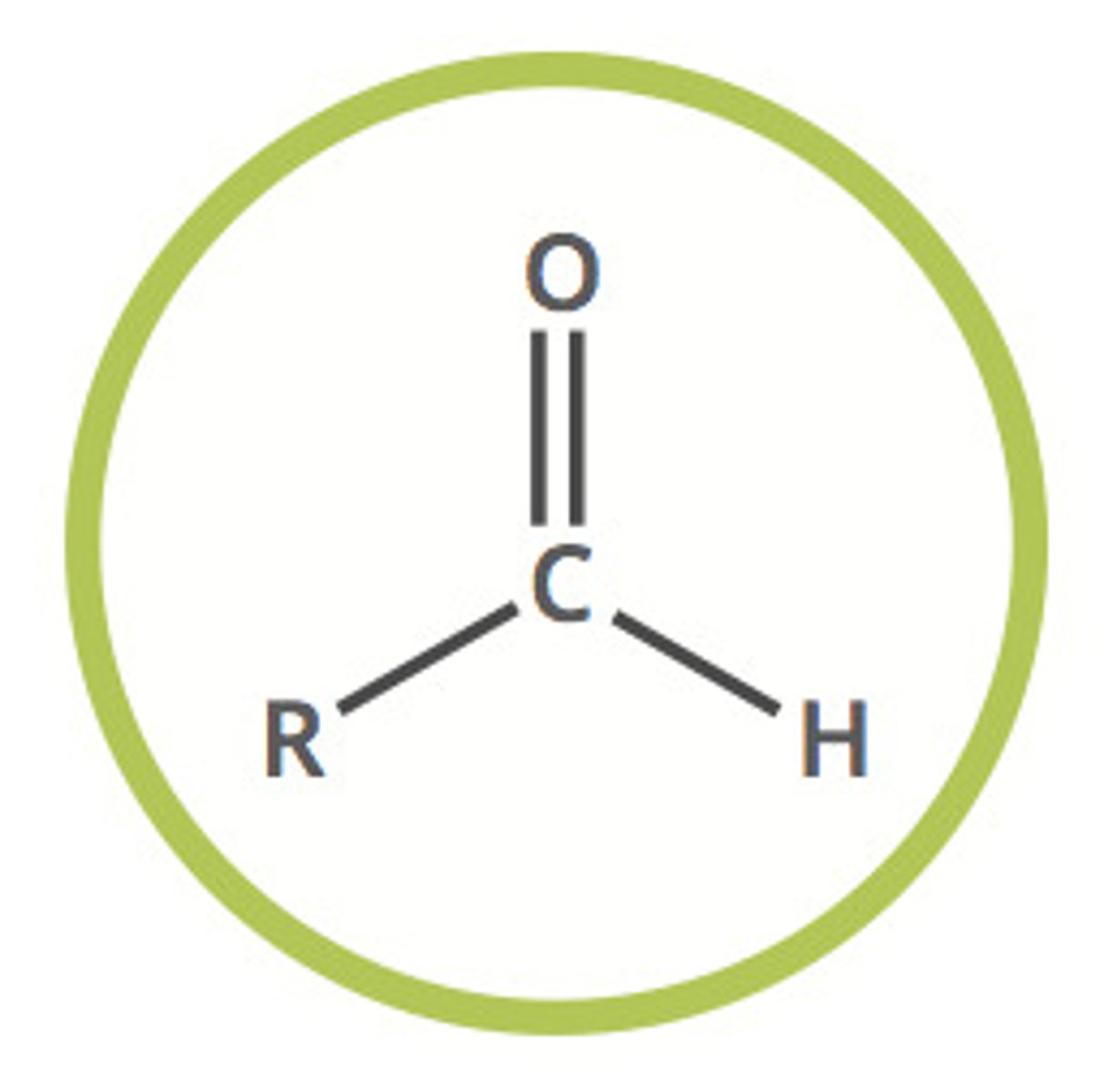
aldehyde
R-CHO
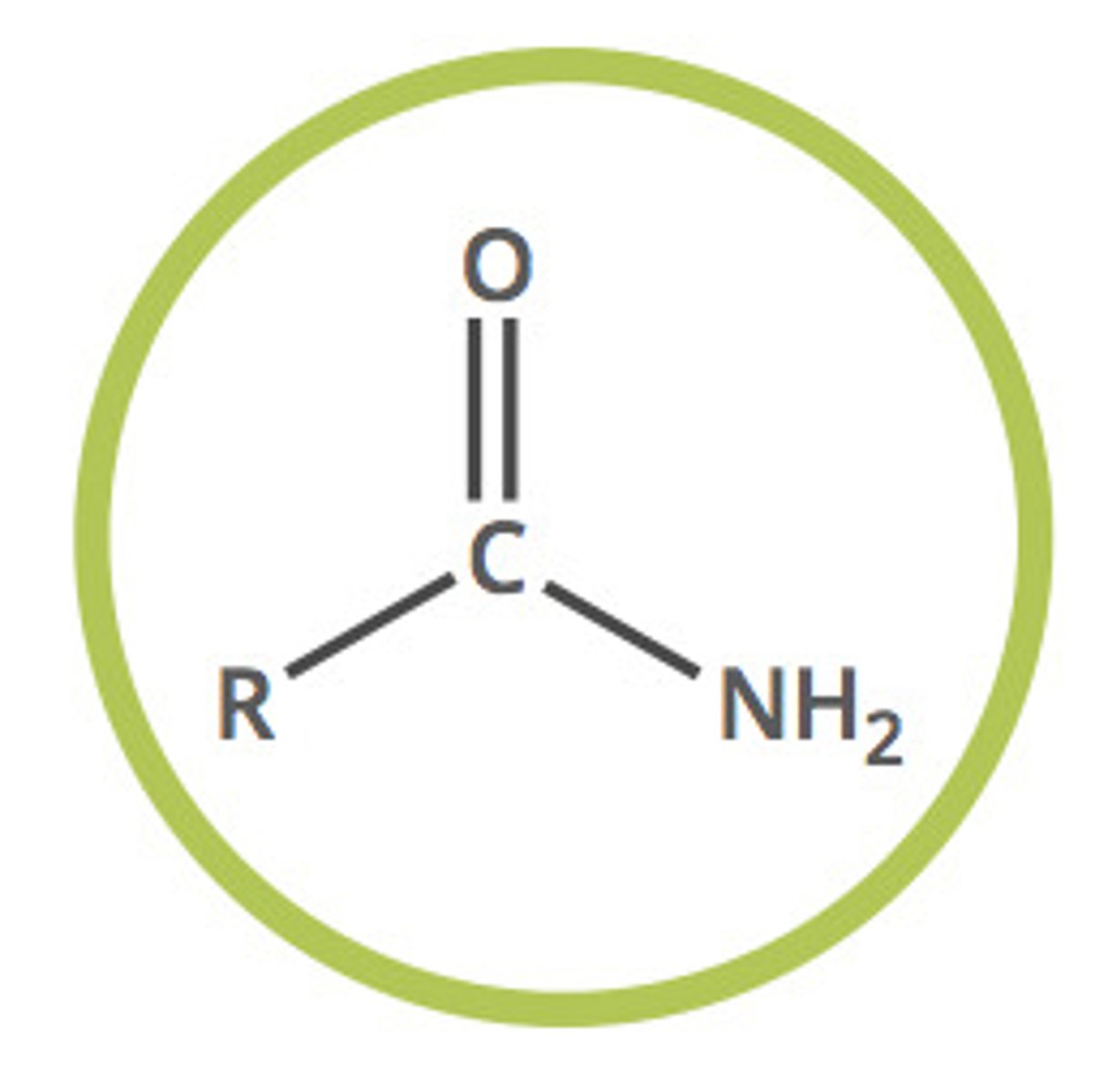
amide
NH2
Which atoms are involved in hydrogen bonding?
H and O, N, or F
ion-dipole attraction
an electrical attraction between an ion and a polar molecule
London forces
the weak attractive forces between all molecules
dipole-dipole attraction
attractive force resulting when polar molecules line up so that the positive and negative ends are close to each other
hydrogen bonding
strong type of intermolecular dipole-dipole attraction; occurs between H and O, N, or F
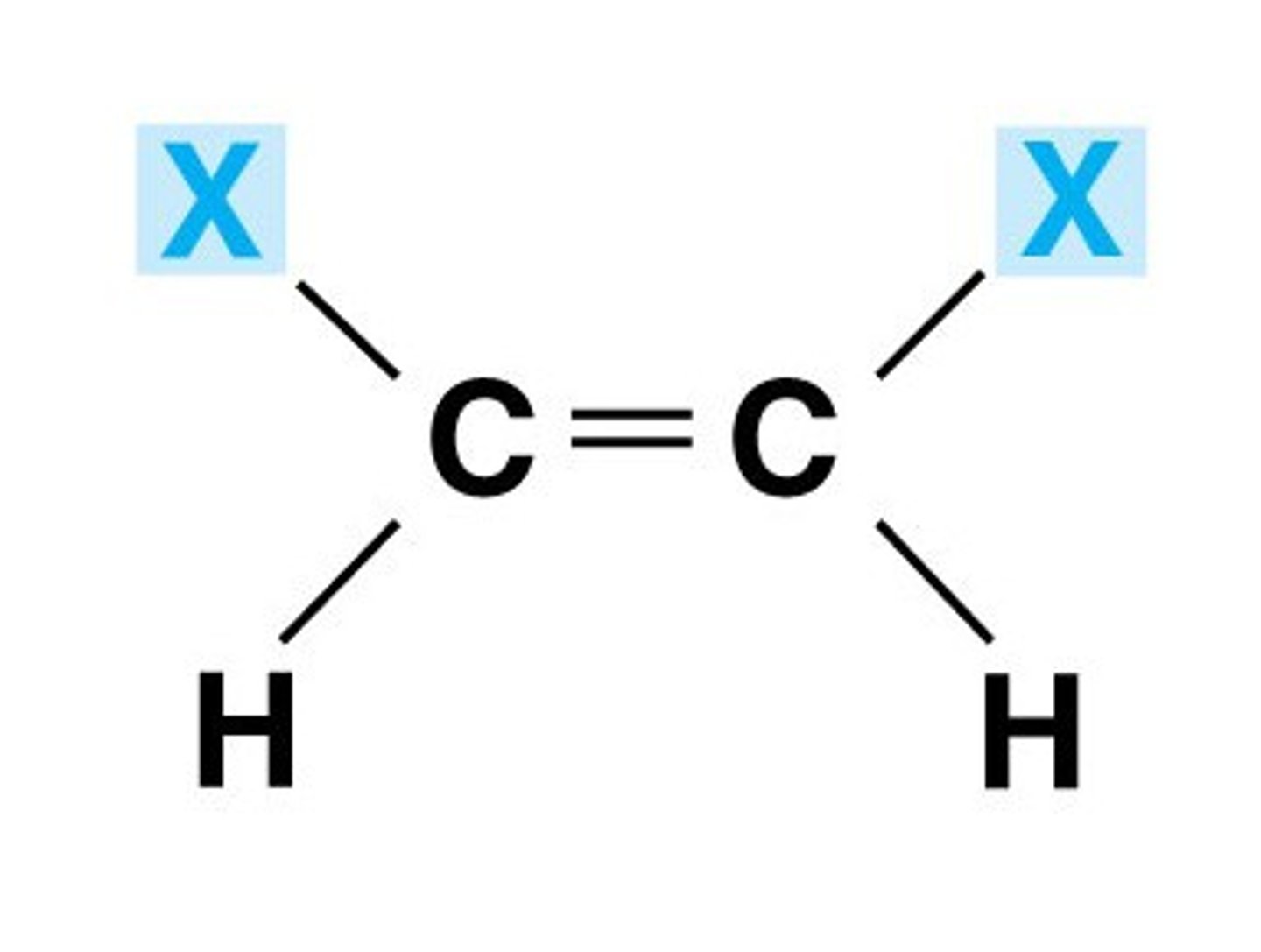
trans bond
hydrogen atoms are positioned on opposite sides of double bond
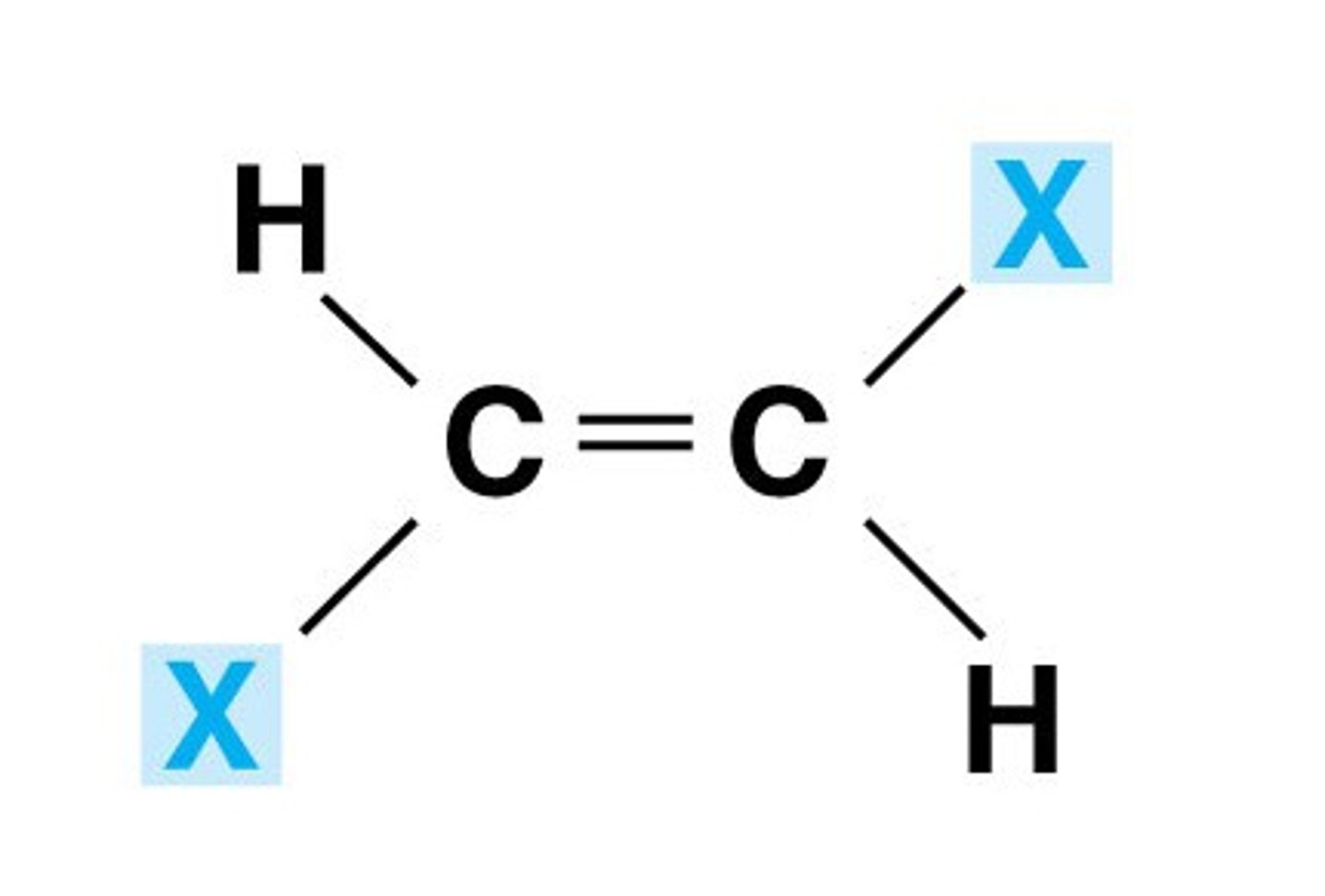
cis bond
hydrogen atoms are positioned on same side of double bond