Quantitative chemistry
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
If a gas escapes during a reaction, the mass will look like it has ______
Decreased
If a gas is gained during a reaction, the total mass will look as though it has _____
Increased
If a thermometer has a mark every 1.0 degrees, what is it’s resolution?
1.0 degrees
Which has a higher resolution, a thermometer with a mark at every 1.0 degrees or one with a mark every 2.0 degrees?
1.0 degrees
The ____ of a measuring instrument is defined as the smallest change in quantity that gives a visible change in the reading
Resolution
The ______ of a measuring instrument is estimated as plus or minus half the smallest scale division
Uncertainty
What is the uncertainty of a thermometer with a mark every 1.0 degrees?
+ or - 0.5 degrees
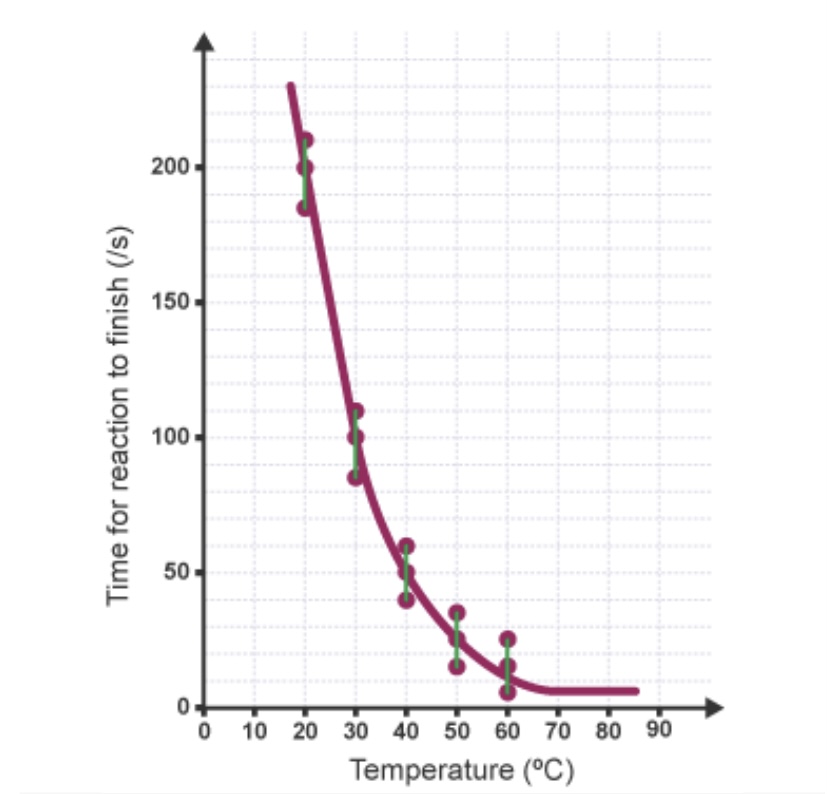
What is represented by the vertical lines that join points on the graph?
The uncertainty
What name is given to the number 6.02 × 10 to the power of 23 ?
Avogado’s constant
What is 6.02 × 10 to the power of 23 equal to?
1 mole
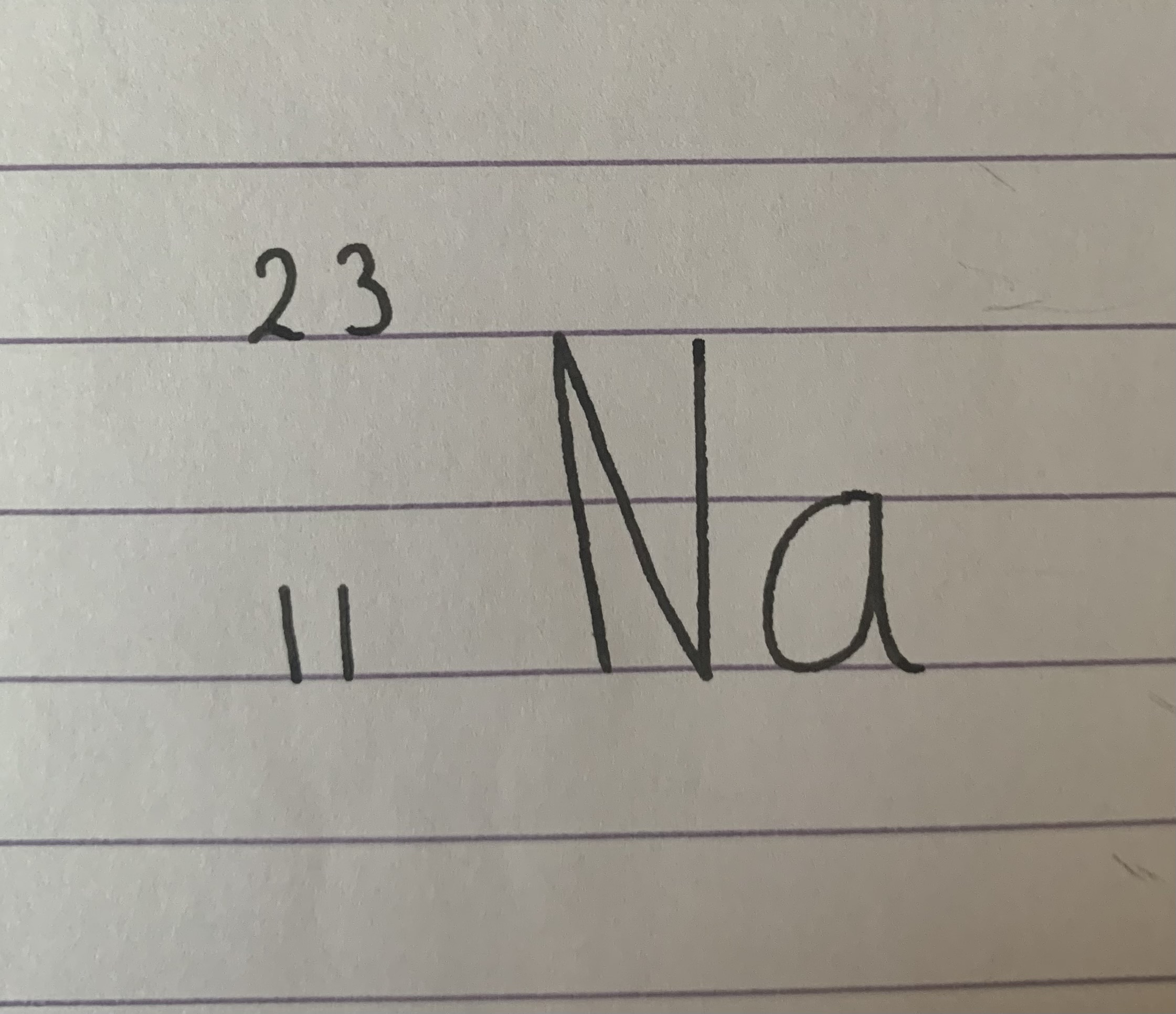
What is the mass number?
23
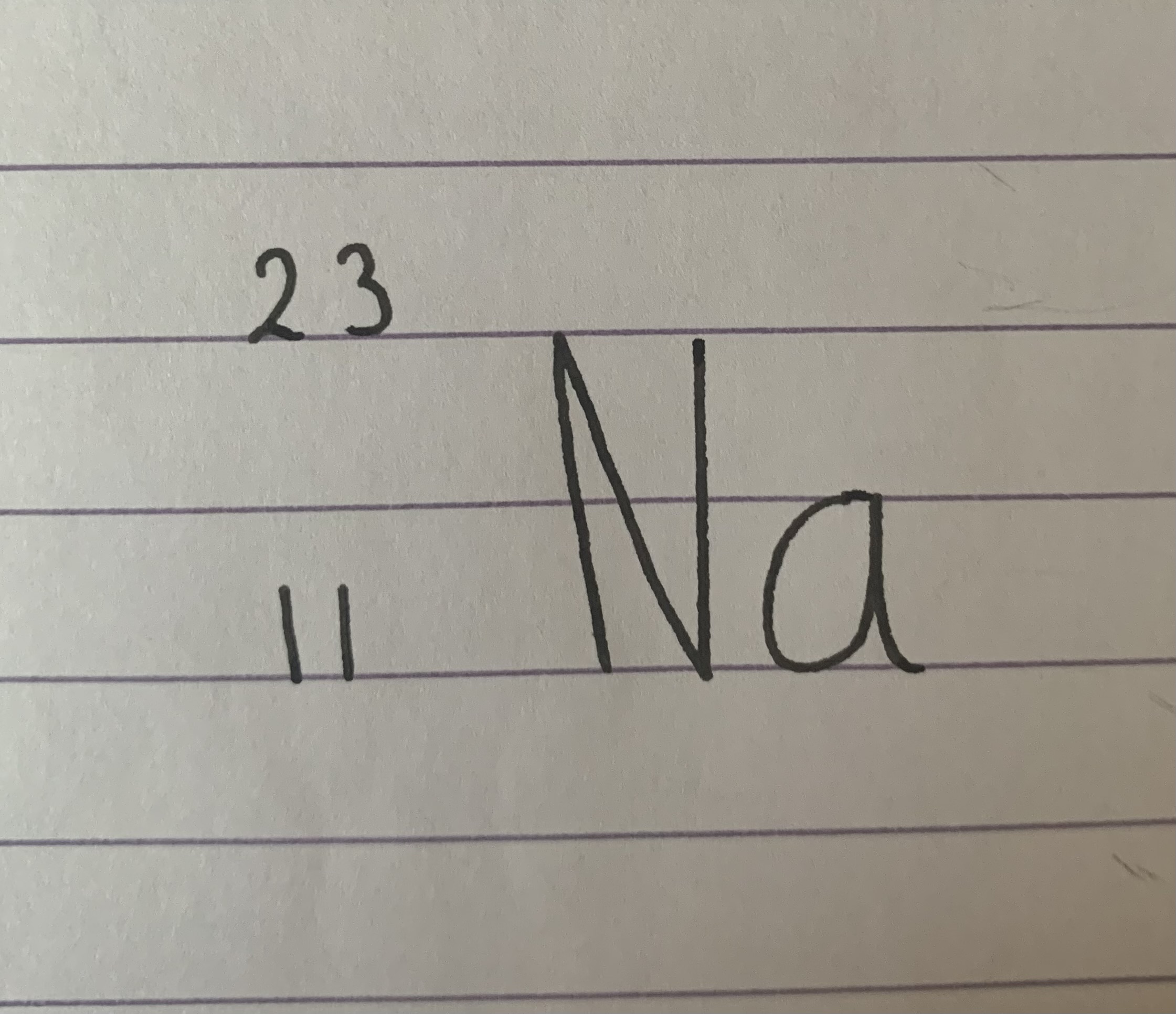
What is the atomic number?
11
What does Mr stand for?
Relative formula mass
What does Ar stand for?
Relative atomic mass
Which law states that no atoms are lost or made in a chemical reaction?
The law of conservation of mass
Which law states that the total mass of products = the total mass of reactants?
The law of conservation of mass
One mole of a substance contains ___ ___ number of particles as one mole of any other substance
The same
Avogadro’s constant x the amount of substance in mol = ….
The number of particles
What is defined as ‘the maximum possible mass of a product that can be made in a chemical reaction’?
The theoretical yield
One reason for not obtaining the calculated amount of a product during a reaction, is that the reaction is reversible and therefore may not go to _____
Completion
One reason for not obtaining the calculated amount of a given product during a reaction is that some product may be lost when it’s ______ from the reaction mixture
Separated
What is the equation for percentage yield?
Mass of product actually made/ maximum theoretical mass of product x 100

Work out the answer
75 percent
Strong acids _____ dissociate into ions in a solution
Completely
Weak or strong? Nitric acid, sulfuric acid and hydrochloric acid
Strong
Weak acids _____ dissociate in solutions
Partially
The higher the concentration of H+ ions in an acidic solution, the ____ the pH
Lower
The lower the concentration of H+ ions in an acidic solution, the ____ the pH
Higher
The higher the concentration of OH- ions in an alkaline solution, the _____ the pH
Higher