Hypersensitivity Lecture
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Hypersensitivity Diseases
Unwanted or misdirected immune reactions
Antibodies that mediate allergies
IgE
Points to remember
Antibodies binding to membrane vs. soluble antigens forming Ag-Ab complexes
Ag-Ab complexes mediate complement activation - lysis and inflammation; neutrophil/macrophage activation (IgM or IgG)
T cells
CD4 T helper cells-cytokines: mediate DTH reaction
CD8 T cells-CTL
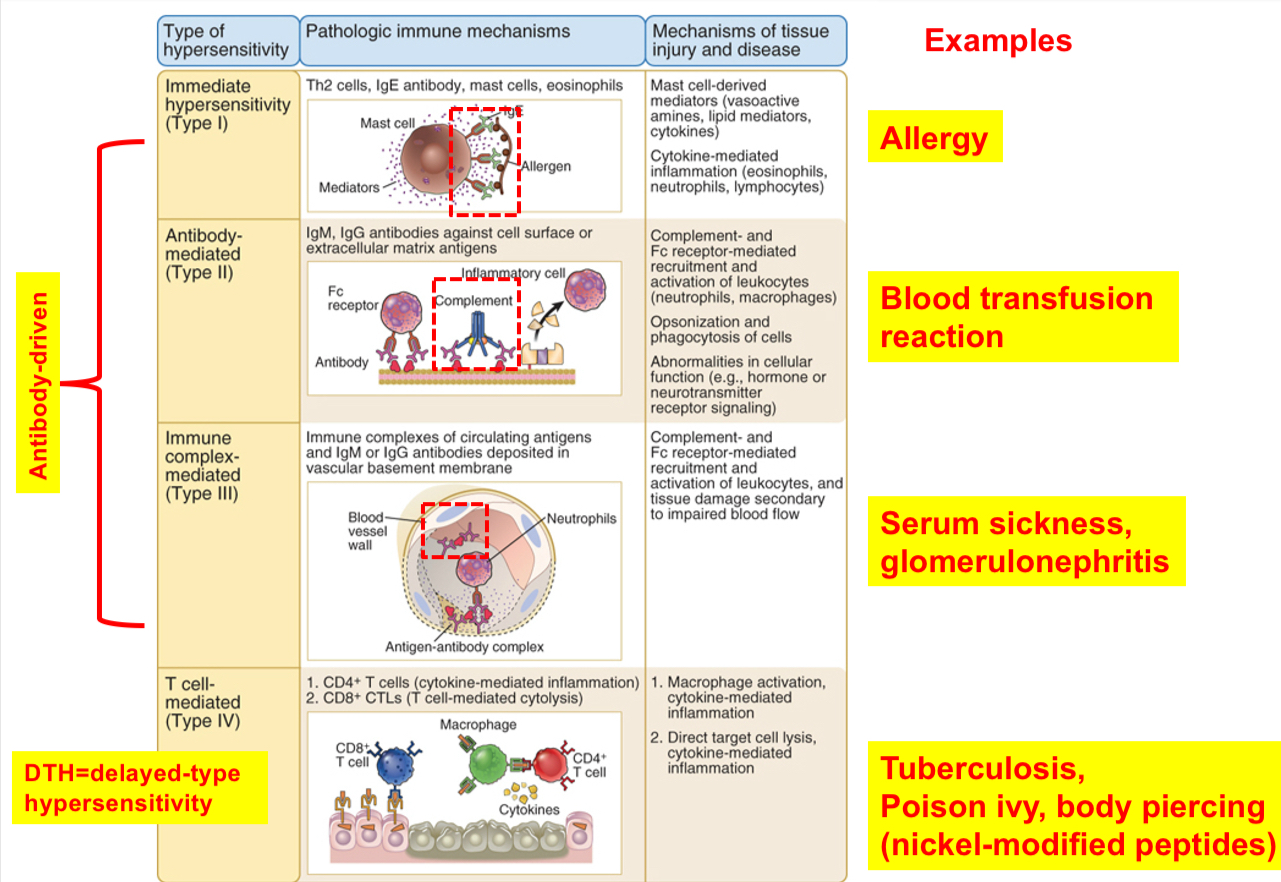
Types of hypersensitivity diseases (Gell and Coombs classification)
(a)Type I or immediate hypersensitivity: IgE antibody against environmental antigens bind mast cells, resulting in the release of mediators (IgE is the mediator)
(b) Type II hypersensitivity: antibody directed against cell or tissue antigens (IgG or IgM are the mediators)
(c) Type III hypersensitivity: antibodies against soluble antigens form complexes, and immune complexes may deposit in blood vessels, causing inflammation and tissue injury (IgG or IgM are the mediators)
(d) Type IV hypersensitivity: T cells can react against foreign Ag or self-tissues (mostly CD4 T helper cells but CD8 T cells can participate)
Mechanism of Type 1 hypersensitivity reaction
•Atopy is allergic response in genetically susceptible individuals; atopic dermatitis is very common in dogs (~15 %).
•It is a rapid, IgE antibody- and mast cell-mediated vascular and smooth muscle reaction and inflammation in individuals exposing to foreign antigens to which they have been previously exposed.
•Allergies account for about 20 % of the most frequent disorders of immune system (humans).
•Dogs: ~ 30 % of skin diseases are allergic dermatitis.
•Common allergic reactions: hay fever, food allergies, bronchial asthma and anaphylaxis
•Mast cells are located in the vascularized connective tissues (called, connective tissue mast cells)-present throughout the body; and those found in the submucosal layers of the gut and respiratory tract are called mucosal mast cells.
Humans:
•7,000 to 20,000 mast cells per cubic millimeter of skin (varies between individuals).
•Mucosal mast cells constitute around 2-3% of all cells within the lamina propria mucosa
Cell types that participate in allergic reactions
Mast cells (major)
Other types: basophils and eosinophils
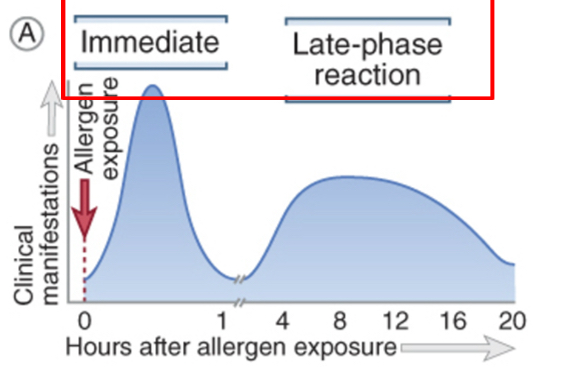
Type l hypersensitivity reactions: immediate and late phases
1. Immediate reaction: cause a rapid increase in vascular permeability and smooth muscle contractions. It can occur within minutes of reexposure to an antigen.
2. Late phase reaction: cytokines secreted by mast cells recruit neutrophils and eosinophils to the site of reaction over several hours. It is responsible for tissue injury that occur due to repeated bouts of immediate hypersensitivity.
Sequence of events in immediate hypersensitivity
Immediate hypersensitivity reactions are initiated by the introduction of an allergen, which stimulates TH2 reactions and immunoglobulin E (IgE) production. IgE binds to Fc receptors (FcεRI) on mast cells, and subsequent exposure to the allergen activates the mast cells to secrete the mediators that are responsible for the pathologic reactions of immediate hypersensitivity.
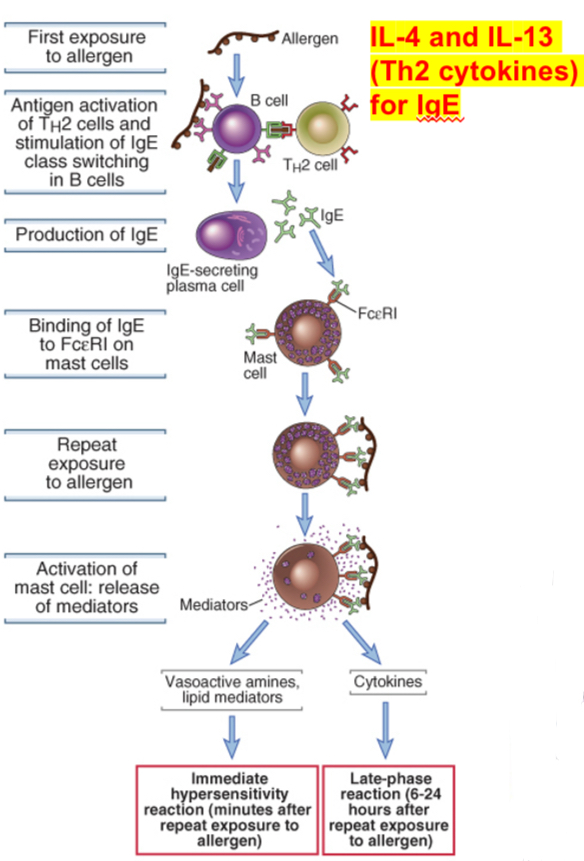
Production of IgE (reaginic antibody)
•Normal individuals: may have small IgE responses but not enough to cause hypersensitivity reactions.
•Mast cells are present in all connective tissues: inhaled allergens activate mast cells in the submucosal tissues of the bronchus; ingested allergens activate mast cells in the intestines.
•IgE binds to FceRI expressed on mast cells: it consists of three chains- one chain binds to Fc portion of IgE strongly (10-11 M).
•Two other chains of the receptor (b and g) are signaling proteins.
•FceRI is also present on basophils (circulating counterpart of tissue mast cells) and eosinophils.
•Antigens that elicit immediate hypersensitivity are called allergens: proteins in pollen, certain foods, insect venoms, animal dander, drugs (e.g. penicillin).
•Upon encountering allergens, Th2 cells get activated; secrete IL-4 and IL-13 and stimulate B cells to switch to IgE-producing cells.
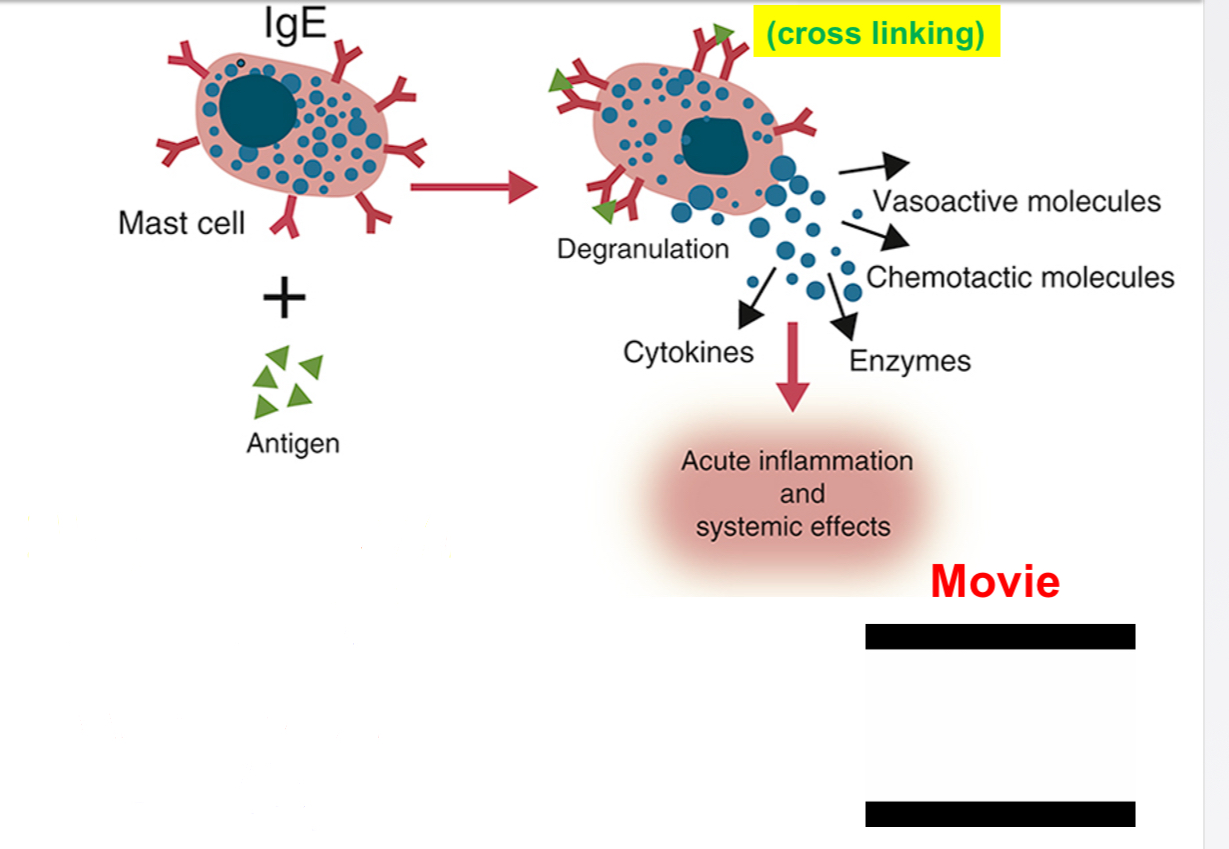
•Activation of mast cells and secretion of mediators:
•IgE antibody binds to high affinity FceR1 expressed on mast cells. Thus in atopic individuals, mast cells are coated with allergen-specific IgE (sensitization). This process makes mast cells sensitive to activation by subsequent encounter with that antigen.
Biochemical pathways in mast cells activation: mediators and their effects
Cross-linking of immunoglobulin E (IgE) on a mast cell by an allergen stimulates phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in the signaling chains of the IgE Fc receptor (FcεRI), which then initiates multiple signaling pathways. These signaling pathways stimulate the release of mast cell granule contents (amines, proteases), the synthesis of arachidonic acid metabolites (prostaglandins, leukotrienes), and the synthesis of various cytokines. TNF, tumor necrosis factor.
Essentially, the polypeptide chain structure of the high-affinity IgE Fc receptor (FcɛRI). IgE binds to the Ig-like domains of the α chain. The β chain and the γ chains mediate signal transduction. The boxes in the cytoplasmic region of the β and γ chains are ITAMs, similar to those found in the T cell receptor complex
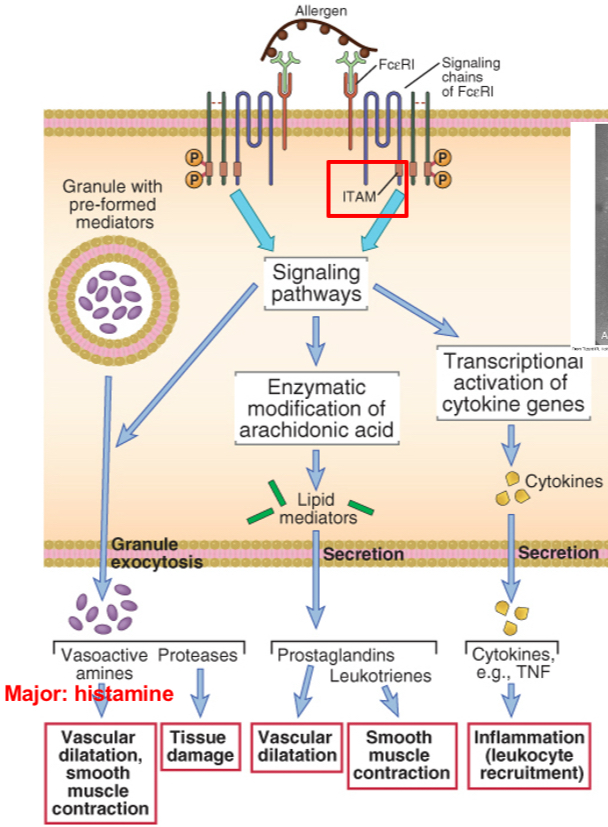
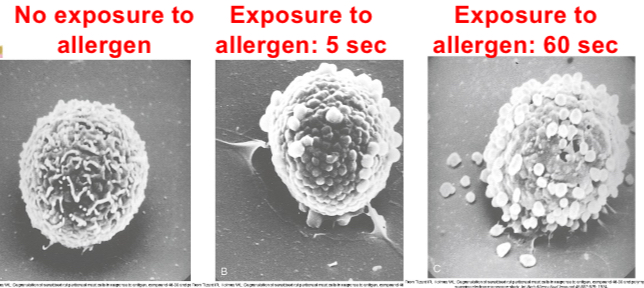
Mast cell reaction: degranulation event
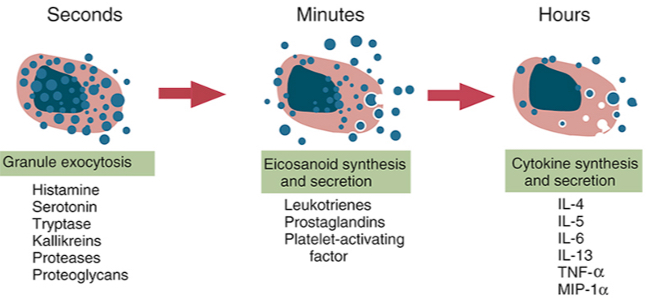
Mast cell reaction: mediators (time-course events)
•Initial exposure elicits IgE production (sensitization); repeat exposure activates sensitized mast cells.
•Mast cell activation occurs when allergen binds to two or more IgE antibodies on mast cells (cross linking) triggering biochemical signals.
•Three types of responses: 1. degranulation: mediators-vasoactive amines and proteases.
•Major amine is histamine: dilatation of small blood vessels, increases vascular permeability and stimulates contraction of smooth muscles.
•Proteases: cause damage to local tissues.
•Lipid mediators-products of arachidonic acid metabolites: prostaglandins cause vascular dilatation; leukotrienes stimulate smooth muscle contraction.
•Cytokines-IL-4 and TNF: induce local inflammation (late phase reaction); stimulate recruitment of leukocytes (eosinophils, neutrophils and Th2 cells).
•Chemokines produced by mast cells also contribute to leukocyte recruitment.
•Eosinophils and neutrophils liberate proteases causing tissue damage; Th2 cells exacerbate the reaction.
•IL-5 produced by Th2 cells and mast cells activate eosinophils.
•Hallmarks of immediate hypersensitivity: acute vascular and smooth muscle reactions and inflammation.
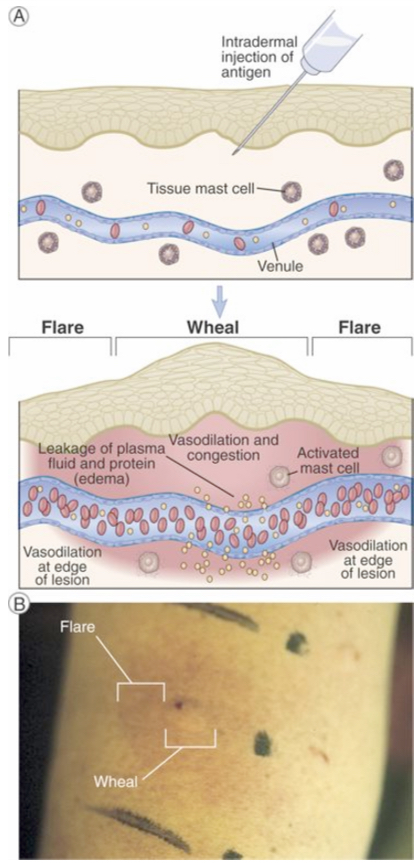
Wheal and flare reaction in the skin
Mast cell mediators → Wheal (redness and local swelling) - dilation of local blood vessels
→ flare (red rim) - dilation of vessels in the edge
In response to antigen-stimulated release of mast cell mediators, local blood vessels first dilate and then become leaky to fluid and macromolecules, which produces redness and local swelling (a wheal). Subsequent dilation of vessels on the edge of the swelling produces the appearance of a red rim (the flare).
B. Photograph of a typical wheal and flare reaction in the skin in response to injection of an allergen.
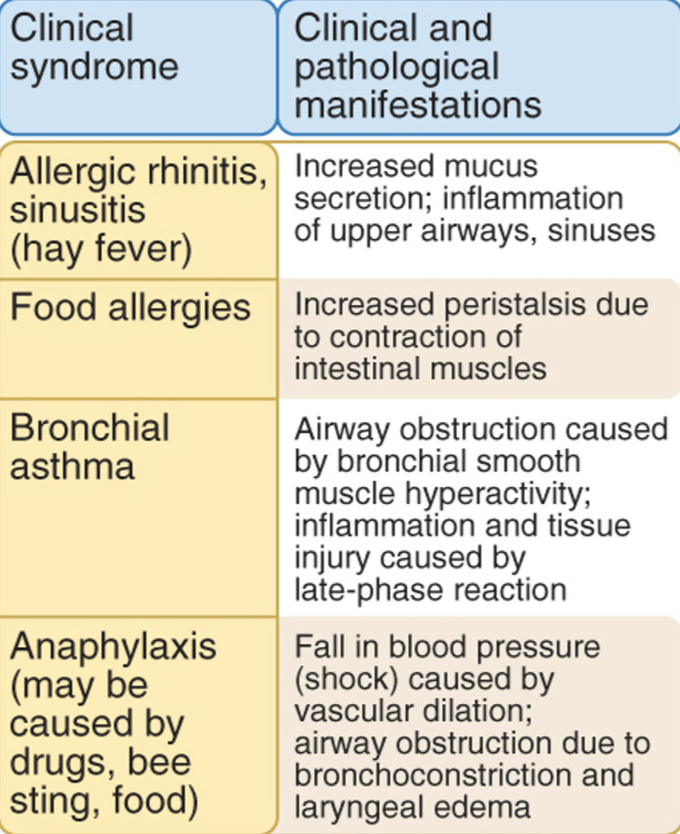
Clinical Syndromes
Hay fever: mast cells in nasal mucosa produce histamine; late phase-prolonged inflammation.
Food allergies: ingested allergens trigger mast cell degranulation; histamine causes increased peristalysis.
Bronchial asthma: respiratory allergy; repeated bouts of bronchial constrictions and airway obstructions
Chronic asthma: eosinophils, excessive mucus secretion in the airways, bronchial smooth muscle becomes hyperactive to various stimuli.
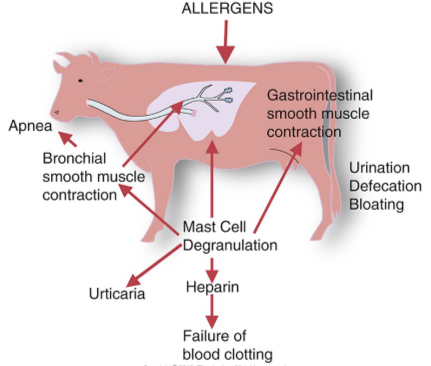
Anaphylaxis
A severe form of immediate hypersensitivity that can be fatal.
Widespread mast cell activation leads to degranulation in response to a systemic antigen.
It is a systemic reaction characterized by edema in many tissues, including larynx and a fall in blood pressure and airway obstruction.
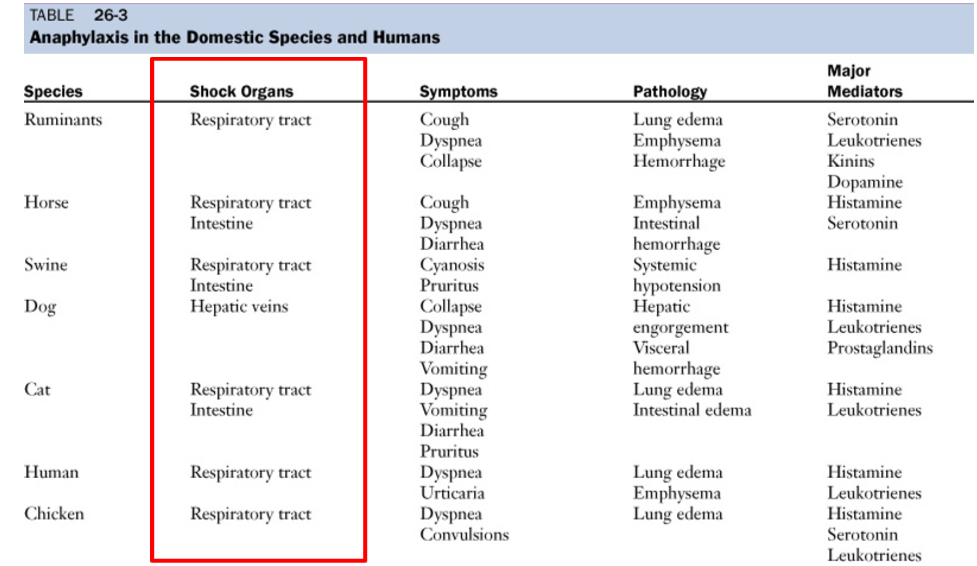
Anaphylaxis in the domestic species and humans
Method to detect type l hypersensitivity reactions: intradermal testing
Small amounts of potential allergens are introduced at specific sites by intradermal injection or by superficial scratching.
Advantage: inexpensive and large number of allergens can be screened
Disadvantages: sensitizes to a new allergen (inviting problem); anaphylactic shock
Treatment of immediate hypersensitivity reactions
Inhibiting mast cell degranulation, antagonizing the effects of mast cell mediators and reducing inflammation.
Antihistamines: relax bronchial smooth muscles in asthma; epinephrine in anaphylaxis.
Corticosteroids: inhibit inflammation.
Humanized monoclonal antibody to IgE (e.g . Omalizumab, approved in the U.S)-useful only if IgE is not already bound to FceR1 e.g. food allergies
Block the action of cytokines e.g IL-4 / IL-5 or their receptors
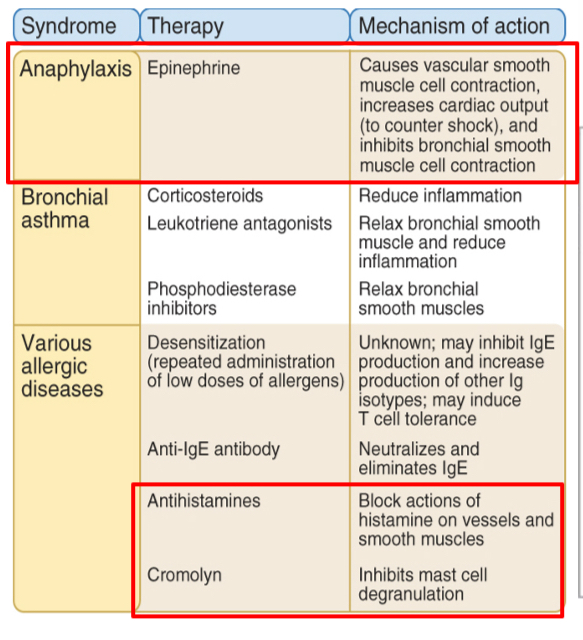
Mediators and treatment of asthma
Mast cell–derived leukotrienes and PAF are thought to be the major mediators of acute bronchoconstriction. Therapy is targeted both at reducing mast cell activation with inhibitors such as cromolyn and at countering mediator actions on bronchial smooth muscle by bronchodilators such as epinephrine and theophylline. These drugs also inhibit mast cell activation.
Mast cell–derived cytokines are thought to be the major mediators of sustained airway inflammation, which is an example of a late-phase reaction, and corticosteroid therapy is used to inhibit cytokine synthesis. Cytokines are also produced by TH2 cells.
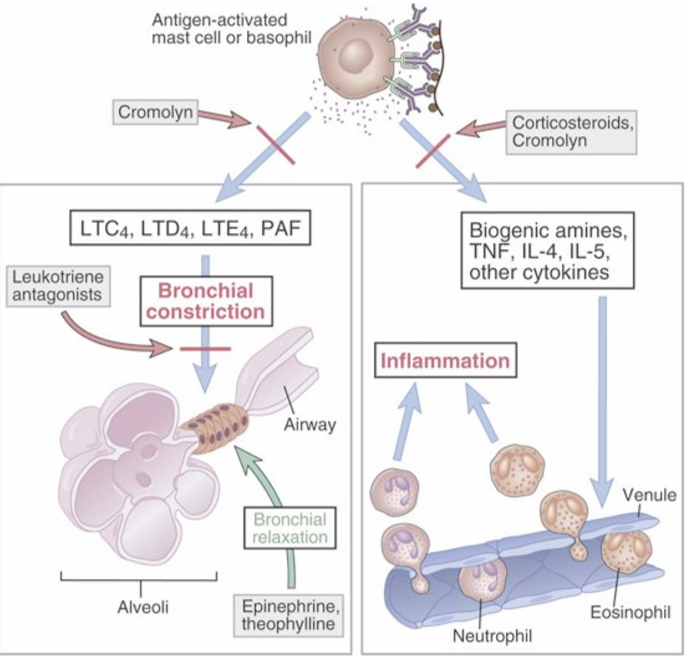
Hyposensitization or desensitization
Increasing doses of allergen could reduce the severity of type l reactions.
Repeated doses cause a shift toward IgG production (blocking antibody) (Th1 response) that turns off IgE.
IgG competes for allergen and forms a complex and allergen is removed by phagocytosis. Thus, allergen is not available to cross-link IgE on mast cells.
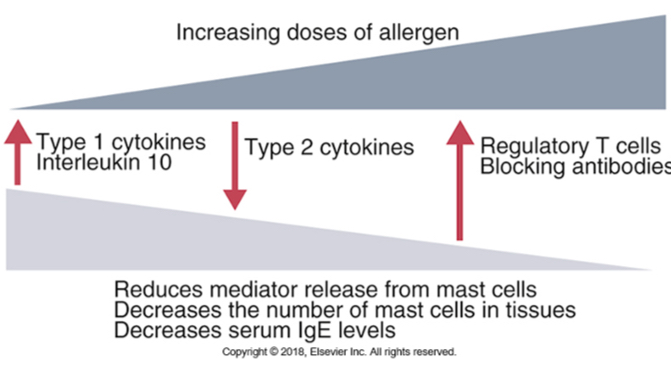
Type ll hypersensitivity reactions: effector mechanisms
Complement activation: inflammation caused by the activation of complement pathway via IgM or IgG binding the tissue antigens (recall anaphylatoxins-complement products and their role in inflammation) (Ex: blood transfusion reactions)
Leukocyte activation: IgG antibodies (IgG1/IgG3) binding to neutrophils and macrophages via FcR activate them to cause inflammation (ROS, lysosomal enzymes)
Opsonization and phagocytosis: antibodies binding to RBCs and platelets can be opsonized and destroyed by phagocytes
Type ll hypersensitivity (antibody-mediated destruction of RBCs): transfusion reactions
RBC antigens:
Blood group antigens - inherited
Glycolipids or glycoproteins on surface of RBCs
Function largely unknown
Vary in structure an antigenicity
No common system between species
Human blood system:
A and B antigens on surface of RBCs
Natural antibodies in plasma to RBC antigens
IgM isotype
Antibodies to blood group antigens are present without previous exposure
Cross reactive epitopes with bacteria that colonize GI tract
Additional RBC antigens: Example, Rh Factor
Rh Factor = + or -
No natural antibody for it
R.R. on RBCs = Rh+
Very immunogenic, stimulates IgG response in Rh- host
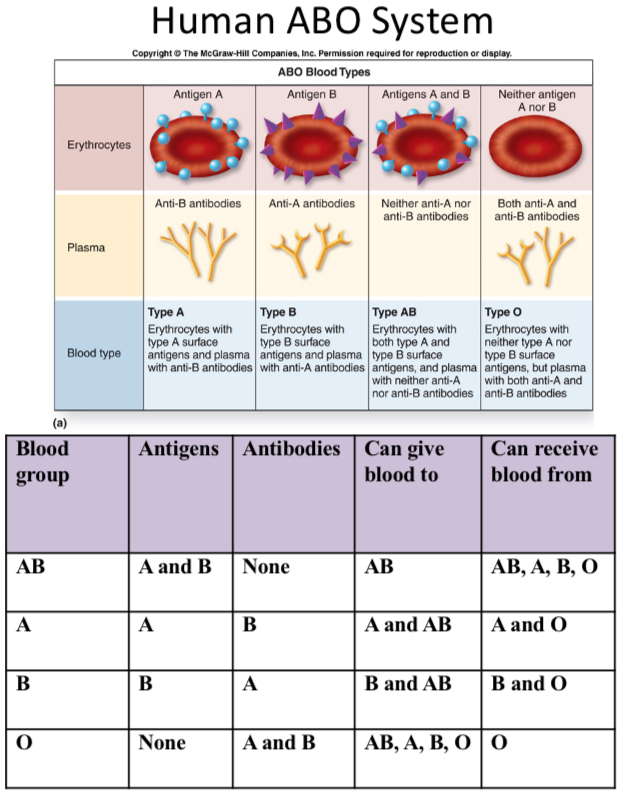
Cat blood types and antibodies to RBC antigens
Blood types = A, B, AB
Natural antibodies:
To A antigen - causes strong hemolysis
To B antigen - causes early cell removal
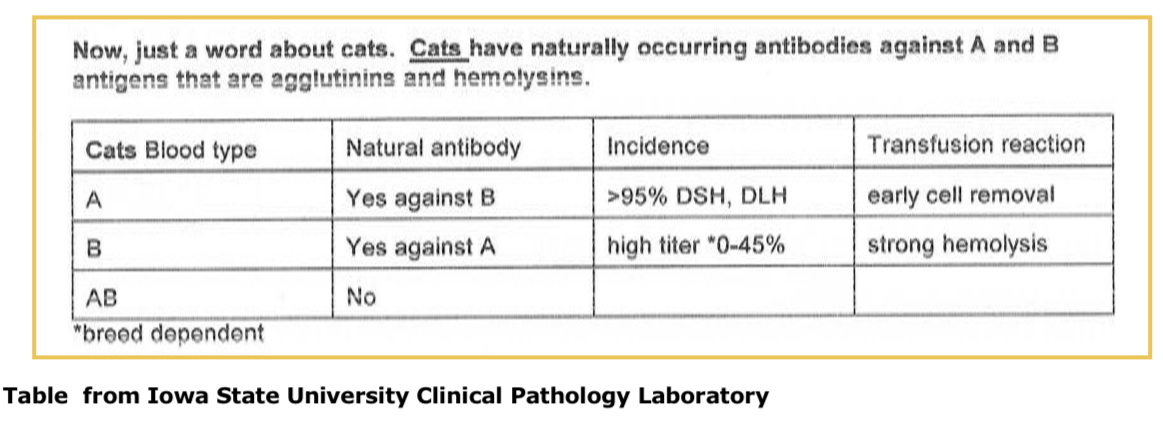
Dog blood types and antibodies to RBC antigens
Natural antibodies - not clinically significant
DEA 1.1 and 1.2 are immunogenic RBC antigens; stimulate IgG production in 1.1 and 1.2 negative animals
Universal donors are 1.1 and 1.2 negative
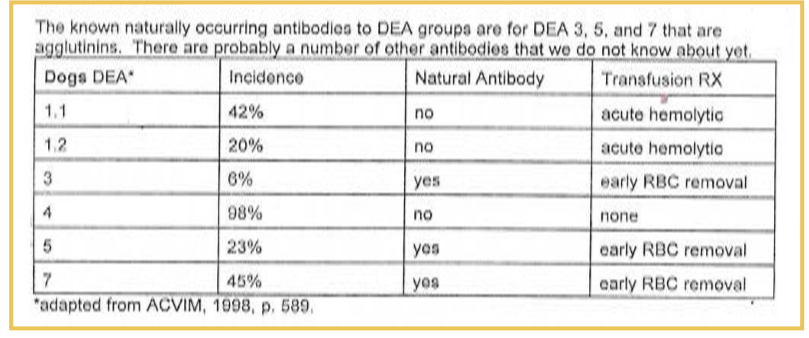
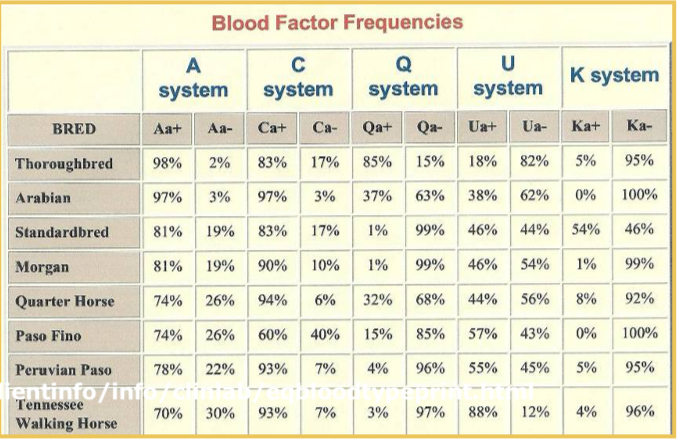
Equine blood types and antibodies to RBC antigens
Seven equine blood types, no clinically significant natural antibody
A and Q are immunogenic, stimulate IgG in A and Q negative animals
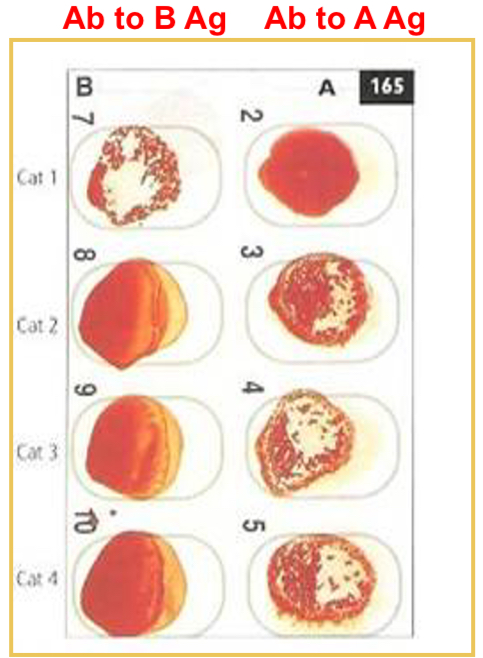
Blood typing: card agglutination
Card contains lyophilized antiserum to the particular RBC antigen
Place 50 microliters of saline and 50 microliters of anticoagulated whole blood on an oval
Mix and rock one minute
Results: Cat 1 is type B, cats 2, 3, and 4 are type A
Cross-matching
Major cross match
Donor cells (washed RBCs) with recipient’s plasma
Procedure
1 drop donor’s cells + 1 drop recipient’s plasma, incubate at 37 degrees C for 30 minutes, look for agglutination or hemolysis
If positive - do not transfuse!
Type ll hypersensitivity: erythroblastosis fetalis
Hemolytic disease of newborn: maternal IgG antibodies specific for fetal blood-group antigens cross the placenta and destroy fetal red blood cells.
Severe hemolytic disease of the newborn called erythroblastosis fetalis develops when a Rh+ fetus expresses an Rh antigen on its blood cells but the mother is Rh-ve
Treatment of erythroblastosis fetalis
RHOGAM: anti-Rh antibodies
Plasmapheresis: mother’s blood is fractionated into cells and plasma
Plasma-containing anti-Rh antibody is discarded and the cells are reinfused into the mother.
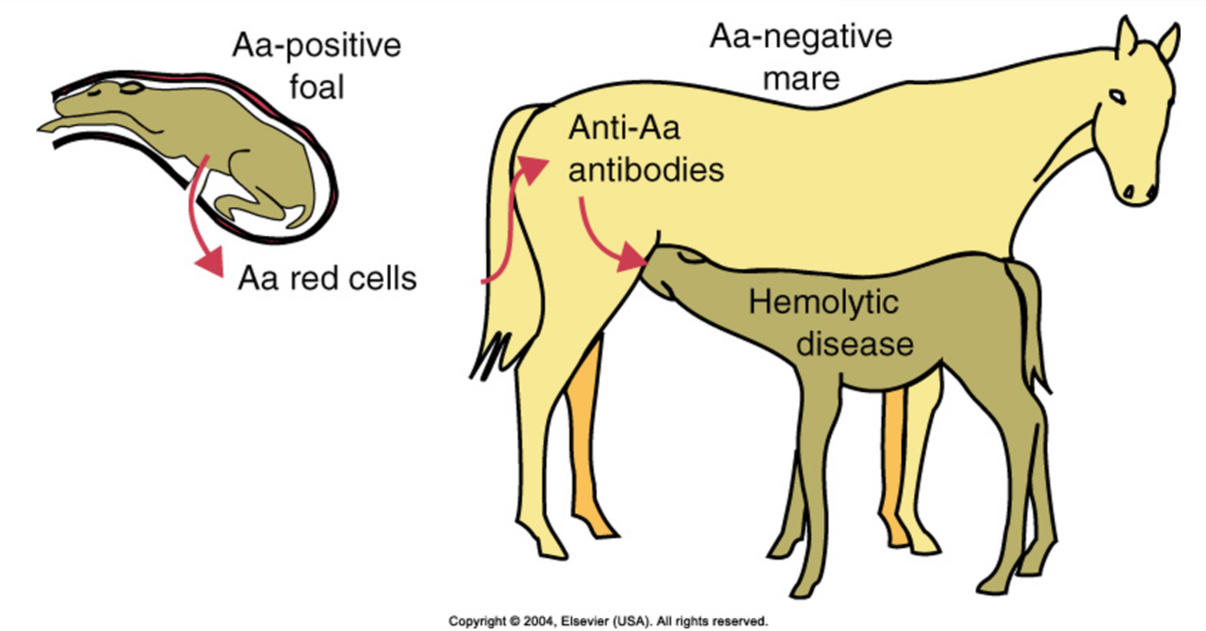
Type ll hypersensitivity: hemolytic disease in foals
First stage: fetal RBCs leak into the mother’s circulation and sensitize her
Second stage: antibodies from the mother are concentrated in colostrum and are then ingested by the suckling foal; ingested antibodies enter the foal’s circulation and cause red blood cell destruction
Pathogenesis of hemolytic disease in foals
•Mare is exposed to a RBC antigen of fetus that she does not have and produces antibody (types Aa and Qa)
•Subsequent pregnancy, a foal with that blood type (inherited from sire)
•Ingestion of colostrum containing antibody to foal RBCs
•Lysis or phagocytosis of foal RBCs
If mare lacks Aa and Qa antigens and the sire has them these are the ones that seem to induce the best immunologic response in the mare. She will produce a lot of antibody to these antigens. The first pregnancy is not usually a problem. The later pregnancies is when the problems occur. The exposure can occur due to a tear in placenta, at birth when there is a tear and exposure of the mother to fetal cells, or transfusion.
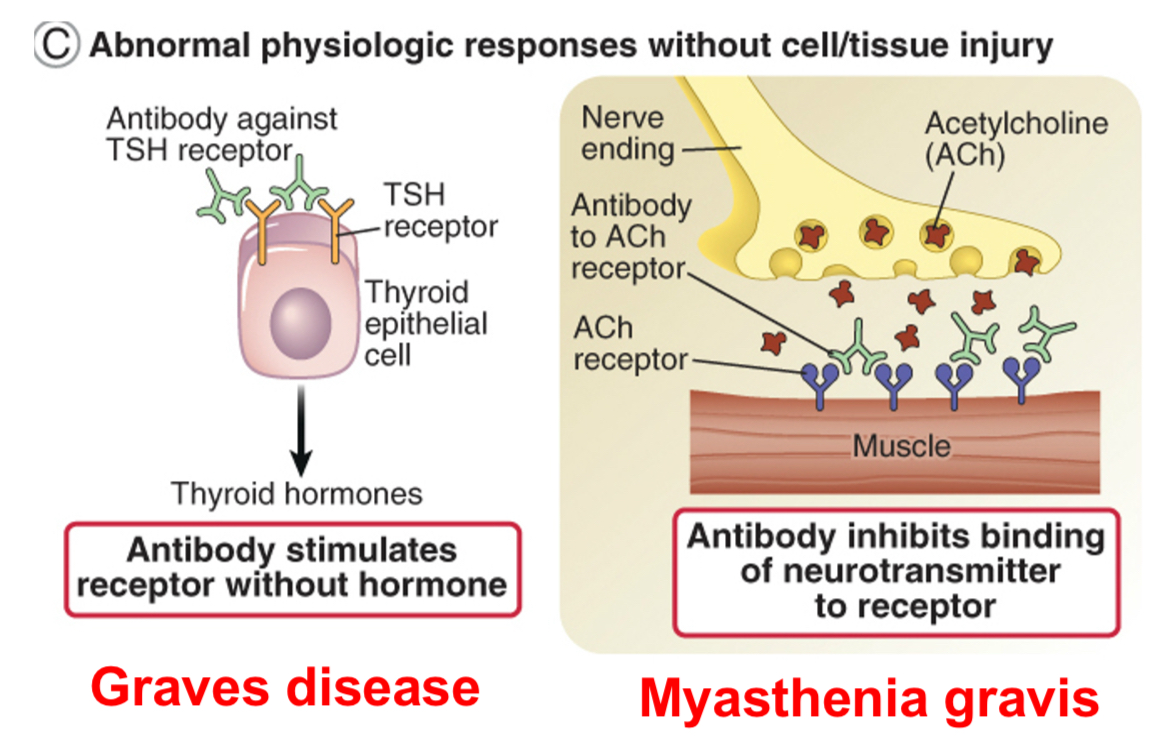
Type ll hypersensitivity: autoantibodies can interfere with normal cellular functions (hormone receptor signaling)
Antibodies may cause disease without inducing tissue injury e.g. anti-acetyl choline receptor antibodies in myasthenia gravis.
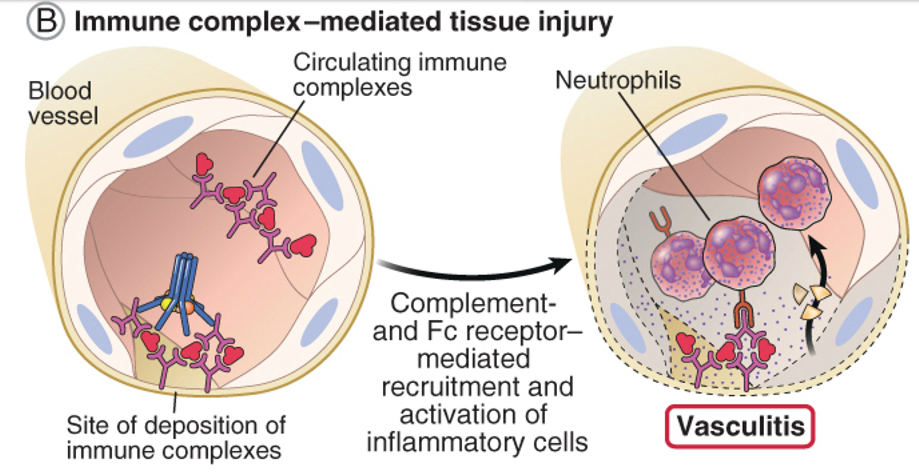
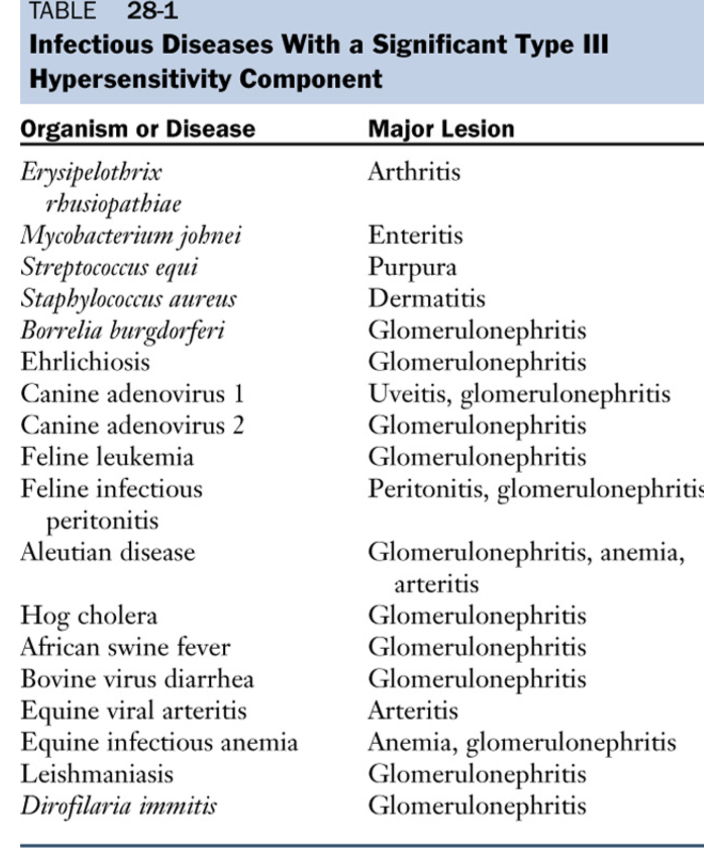
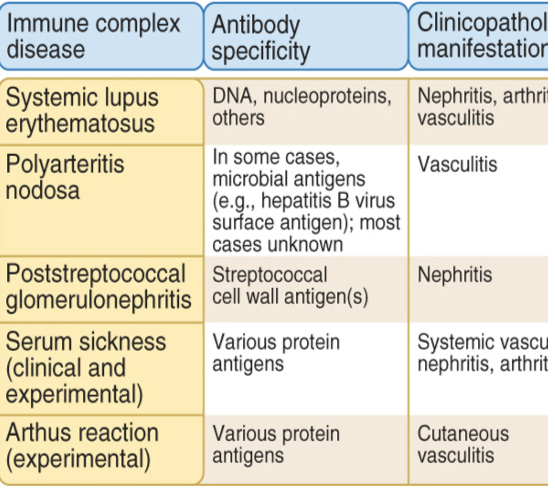
Type lll - Immune complex-mediated hypersensitivity
Largest amounts of immune complexes can lead to tissue-damaging type lll reactions.
Local: complexes deposited at the site of antigen entry cause localized reaction
Systemic: reactions can also develop wherever the complexes are deposited in blood vessel walls, synovial membranes in joints, glomerular basement membrane in the kidney, and choroid plexus in brain, initiating the neutrophils and granular release.
Immune complexes initiate the complement system.
Additional notes on type lll immune complex-mediated hypersensitivity
Antibodies (other than IgE) may cause tissue injury and disease by forming immune complexes that deposit mainly in blood vessels (type III hypersensitivity).
Immune complexes tend to deposit in blood vessels at sites of turbulence (as in branches of vessels) or high pressure (kidney glomeruli and synovium). Tends to be systemic and manifest as vasculitis, arthritis and nephritis.
Autoantibodies may form immune complexes with circulating self antigens.
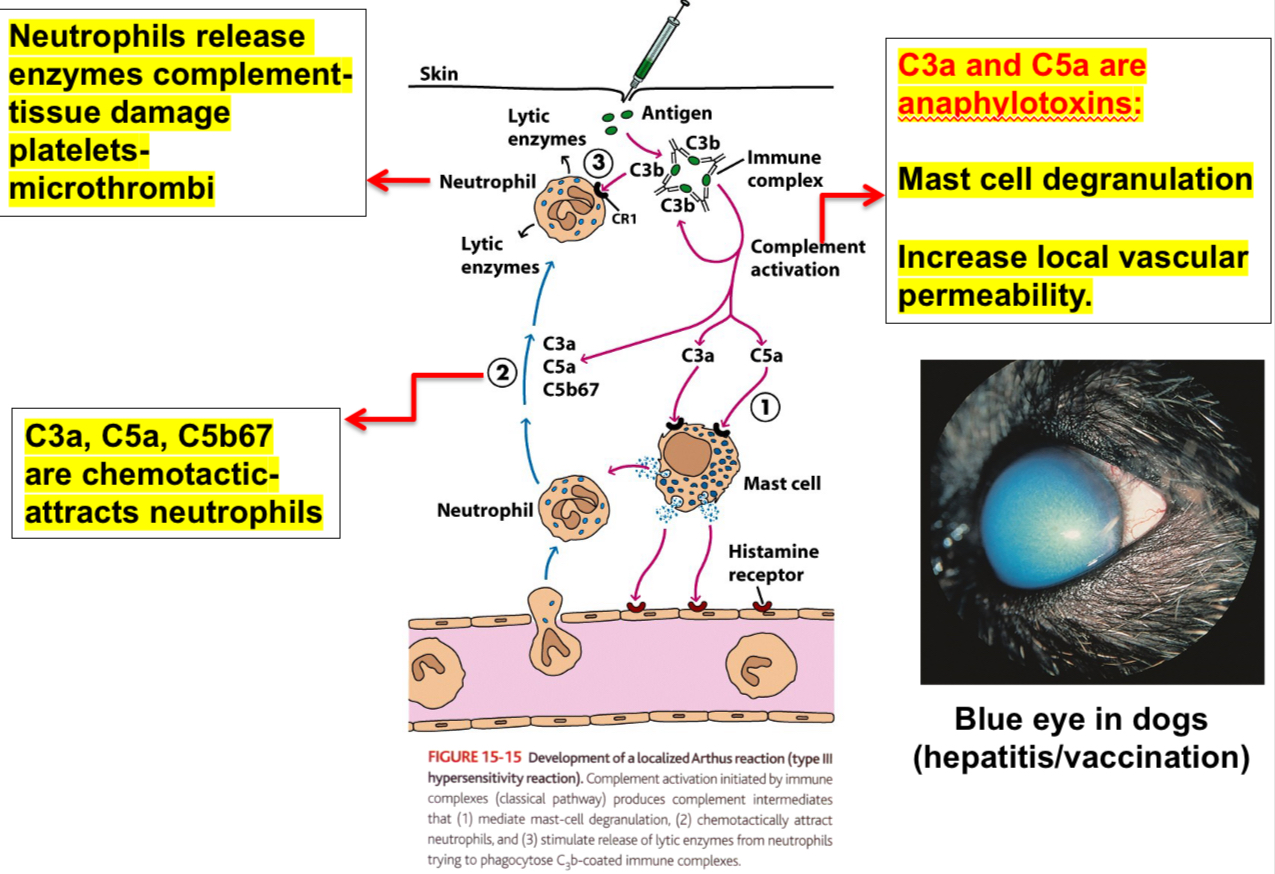
Type lll reactions can be local: Arthus reaction
Injection of an antigen intradermally or subcutaneously into an animal that has high levels of circulating antibody specific for that antigen leads to the formation of immune complexes, and they mediate Arthus reaction within 4-8 hrs.
Neutrophils adhere to the vascular endothelium and then migrate into the tissues at the site of immune complex deposition, leading to vascular damage (edema, erythema).
Examples:
1. Insect bites: can cause Type I reactions initially followed by Type III reactions.
2. Farmer’s lung disease: results from inhalation of thermophilic actinomycetes from moldy hay.
3. Pigeon fancier’s disease: due to inhalation of a serum protein in dust derived from dried pigeon feces. Both cause intrapulmonary Arthus-type reactions (local).
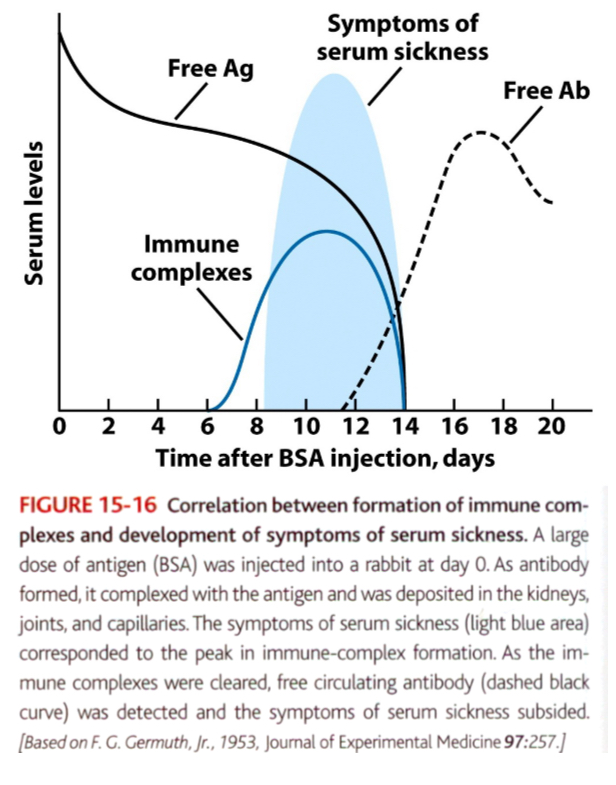
Type lll reactions can be generalized: serum sickness
When large amounts of antigen enter the bloodstream and bind to antibody, circulating immune complexes can form.
If the antigen is significantly excess compared to antibody, the immune complexes that form are smaller.
Because these immune complexes are not easily cleared by phagocytic cells, they can deposit and cause tissue-damaging type lll reactions at various sites.
Ex: administration of antitoxins containing foreign serum such as horse anti-tetanus or anti-diphtheria serum
The recipient develops antibodies specific to foreign serum proteins, and they form circulating immune complexes with the foreign serum antigens.
Within days/weeks after exposure to serum, symptoms of serum sickeness are manifested.
Vasculitis (rashes), arthritis, glomerulonephritis (immune complexes are deposited in tissues where filtration of plasma occurs).
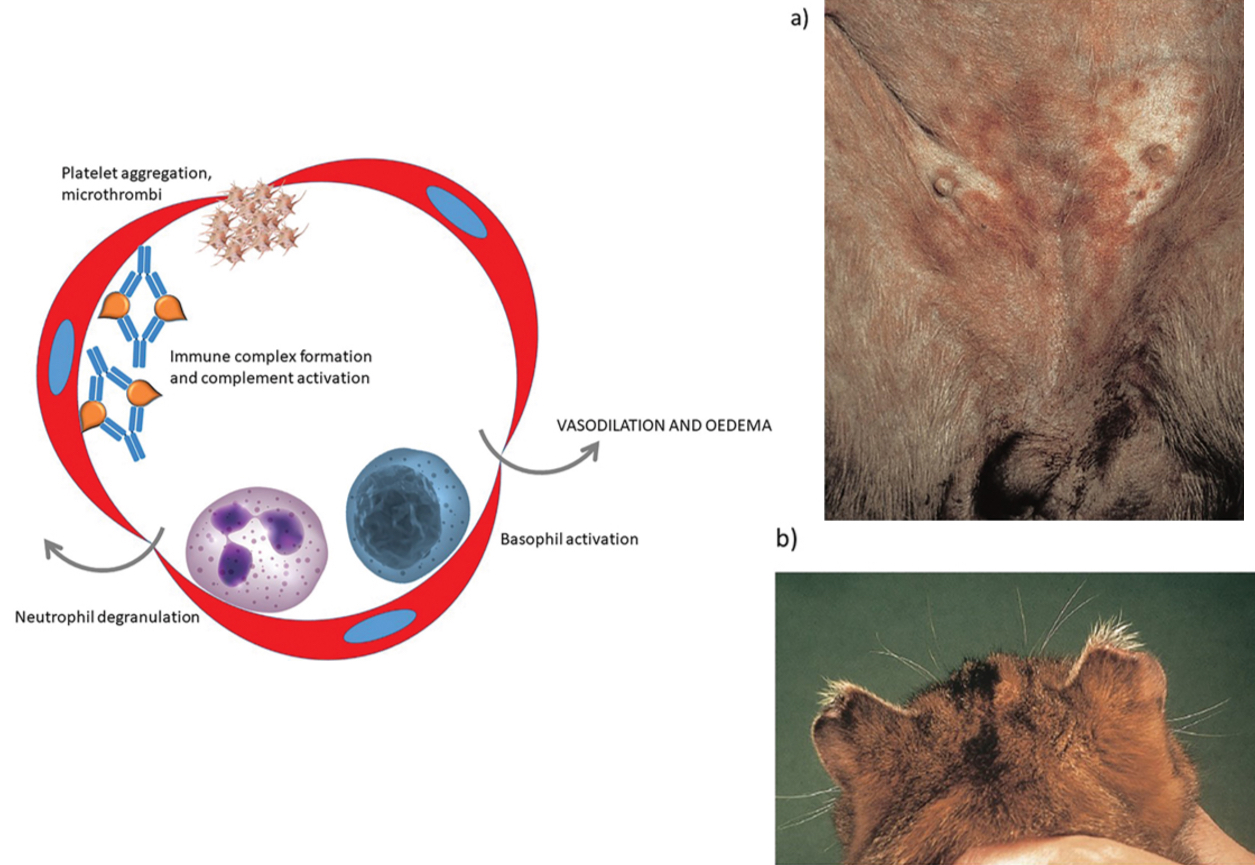
Antigen excess type lll hypersensitivity causes vasculitis
In this form of hypersensitivity, a high concentration of antigen leads to the formation of small, soluble immune complexes. In areas of turbulent blood flow, these are forced against the walls of capillaries and may lodge at these sites. Complement activation results in recruitment of neutrophils with subsequent inflammation within the vessel wall (vasculitis). Local platelet aggregation may result in the formation of microthrombi and ischaemic necrosis of tissue supplied by the vessel. Platelets and basophils release vasoactive amines to cause vasodilation and increased vessel permeability.
(a) The ventral abdomen of this dog has numerous discrete round ‘target’ lesions consistent with vasculitis. This dog had septicaemia and it is likely that immune complexes of bacterial antigen and antibody had formed and deposited within the walls of cutaneous capillaries.
(b) The tips of the ears of this cat have become necrotic and detached. The underlying cause was vasculitis with thrombosis of small capillaries and ischaemic necrosis of the skin.
Veterinary species
Human species
Type ll and type lll hypersensitivity: therapy
Plasmapheresis: to reduce the levels of circulating antibodies to immune complexes
CD40 blockers: inhibit T helper cell-dependent B cell activation
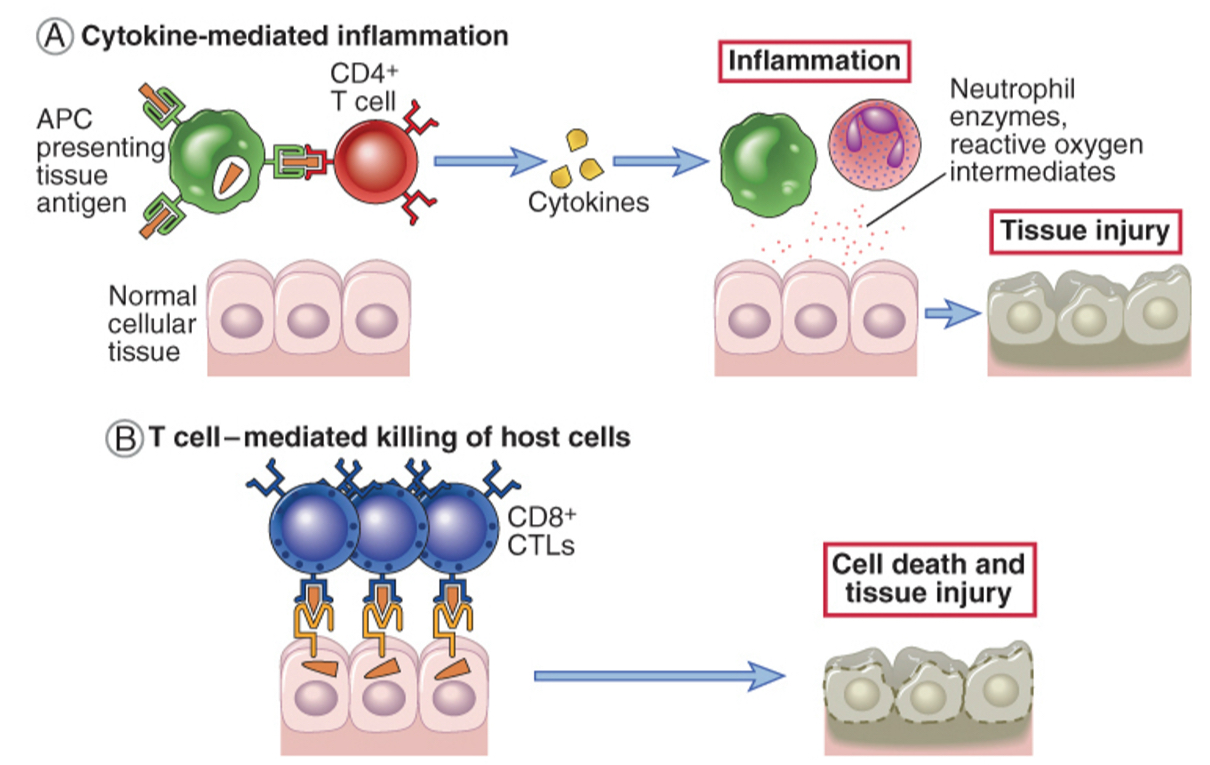
Type lV hypersensitivity (delayed-type hypersensitivity reaction): T cells mediate tissue injury
T cells may cause tissue injury and disease by two mechanisms:
Inflammation may be triggered by cytokines produced mainly by CD4+ T cells and in which tissue injury is caused by activated macrophages and inflammatory cells; APC, Antigen-presenting cell.
Direct killing of target cells is mediated by CD8+ cytotoxic T lymphocytes (CTLs).
DTH reaction is an underlying mechanism of granulomatous inflammation
Remember 2 cells types:
T cells and macrophages (CD4 T cells secrete cytokines that induce local inflammation and activate macrophages). CD8 T cells can contribute to the DTH reaction, B cells have no role.
Granulomatous inflammation
Cytokines are involved in the generation of Th1 cells, activation of macrophages, and recruitment of leukocytes. Prolonged reactions of this type lead to the formation of granulomas.
The pathogenesis of allergic contact dermatitis
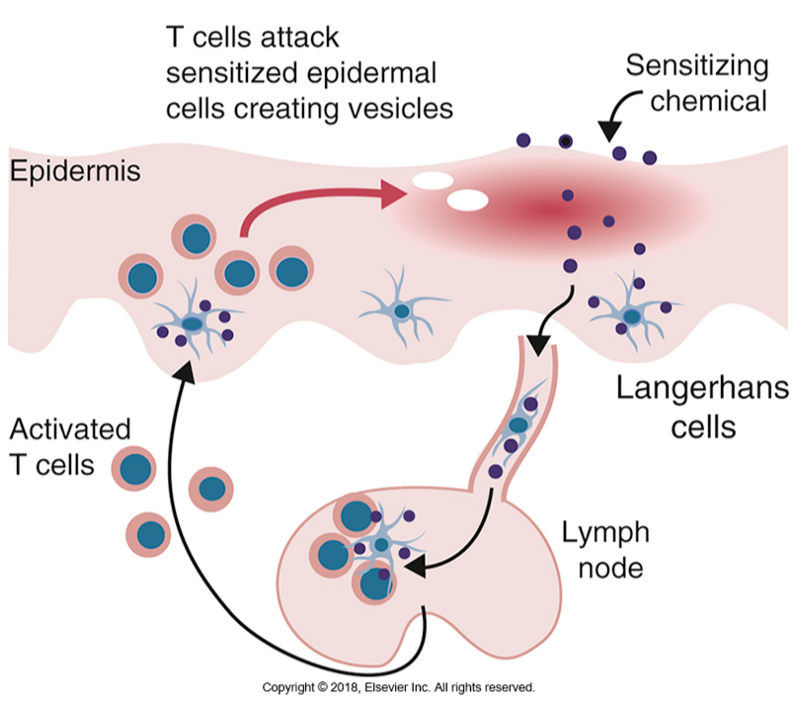
Contact dermatitis induced by poison ivy is a DTH reaction
Small molecule (haptens) can complex with skin proteins (carriers) and are internalized by antigen presenting cells in skin (Langerhan’s cells) which the induce Th1 response.
Example: poison oak leaves contain pentadecacatechol (hapten) forms a complex with skin proteins and induce Th1 response (sensitization phase).
After 48-72 hours after the second exposure, the secreted cytokines cause macrophages to accumulate at the site.
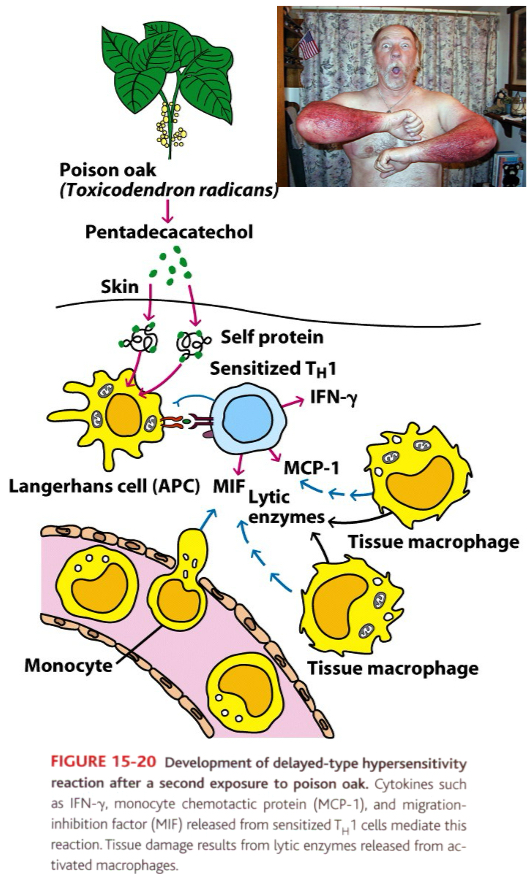
DTH reaction, a measurement of CMI response
Induration is the diagnostic feature (24-48 hours)
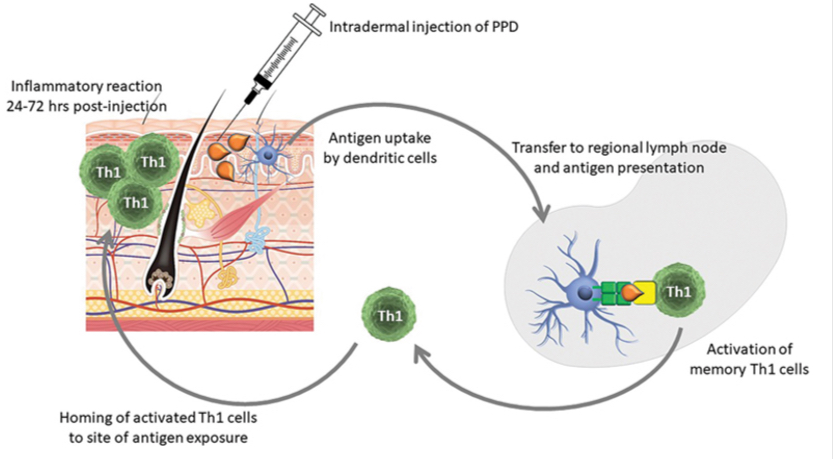
Diagnosis and surveillance for M. bovis (TB test)
Intradermal TB test part of eradication program
Tuberculin = purified protein derivative (ppd) of Mycobacterium
Injection of tuberculin intradermally
Unsensitized animal = no reaction
Sensitized animal = red, indurated swelling at injection site 24-72 hours post injection
Caudal tail fold intradermal injection of tuberculin (ppd) - a protein from M. bovis
Check site at 72 hours for induration
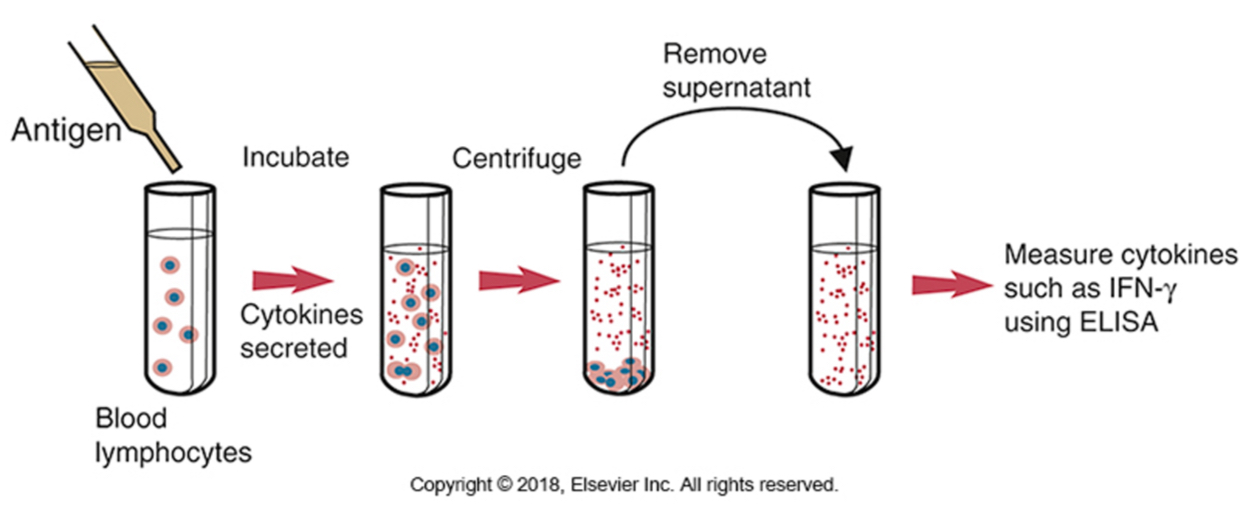
IFN-y release assay (IGRA)-sensitive test: measure IFN-y in response to M. tb antigens ( to diagnose latent TB infections)
The release of IFN-γ by peripheral blood lymphocytes following exposure to tuberculin or to purified mycobacterial antigens. This technique can be used for the diagnosis of tuberculosis in cattle and deer. Tuberculin PPD is added to blood, and the mixture is incubated for 24 to 48 hours. The plasma is then removed and assayed for any interferon produced.
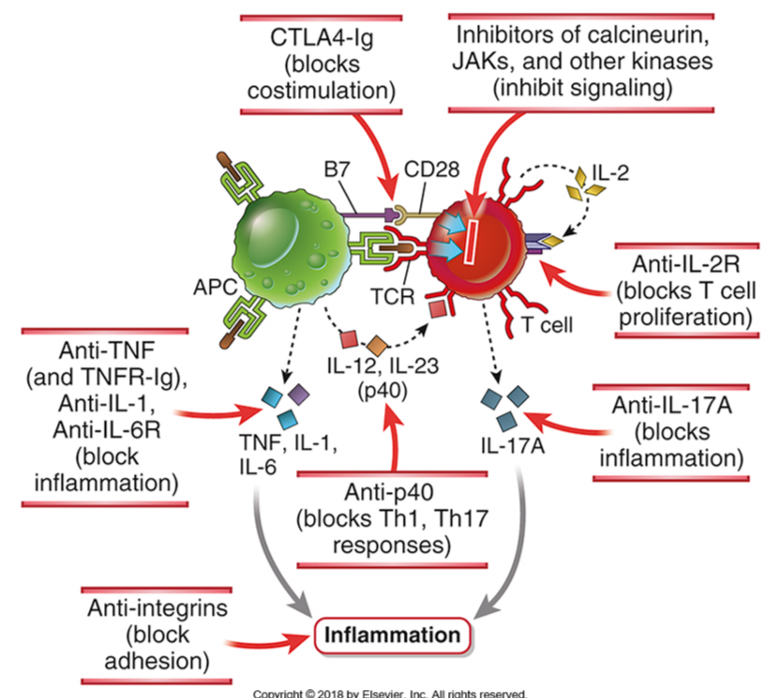
Therapy
Reduce inflammation: by corticosteroids and cytokine antagonists (TNF)-rheumatoid arthritis and IBD
Inhibit T cells responses: by immunosuppressants (cyclosporine)
T cell inhibitors: antagonists to IL-2, B7-blockers
Induce T cell tolerance
Novel therapies for inflammatory diseases targeting T cell responses and inflammation