Quiz 4: Biomedical Sciences - Haskell-Luevano
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
99 Terms
What is the main goal of gluconeogenesis?
To synthesize glucose from non-carbohydrate precursors
How is gluconeogenesis similar to glycolysis?
many enzymes and intermediates are shared
How is gluconeogenesis different from glycolysis?
gluconeogenesis bypasses three irreversible steps of glycolysis using unique enzymes
What are the three major non-carbohydrate precursors for gluconeogenesis?
Lactic acid, amino acids (ex., alanine), and glycerol
Which organ is the major site of gluconeogenesis?
The liver
Which organ plays a smaller role?
The kidney
Does gluconeogenesis occur significantly in brain, skeletal muscle, or heart muscle?
No, very little
- these tissues consume glucose rather than produce it
Is gluconeogenesis simply the reversal of glycolysis?
No, it uses bypass reactions, making it energetically favorable (-9 kcal/mol)
- reversal of glycolysis would require too much energy (+20 kcal/mol).
What are the three irreversible enzymatic steps in glycolysis?
Glucose → Glucose-6-phosphate (hexokinase)
Fructose-6-phosphate → Fructose-1,6-bisphosphate (phosphofructokinase)
Phosphoenolpyruvate → Pyruvate (pyruvate kinase)
What are the three irreversible steps of gluconeogenesis?
Pyruvate → Oxaloacetate → Phosphoenolpyruvate (pyruvate carboxylase + PEP carboxykinase)
Fructose-1,6-bisphosphate → Fructose-6-phosphate (fructose-1,6-bisphosphatase)
Glucose-6-phosphate → Glucose (glucose-6-phosphatase, in ER)
Where does the pyruvate carboxylase reaction occur?
in the mitochondria
What cofactor does pyruvate carboxylase require?
Biotin
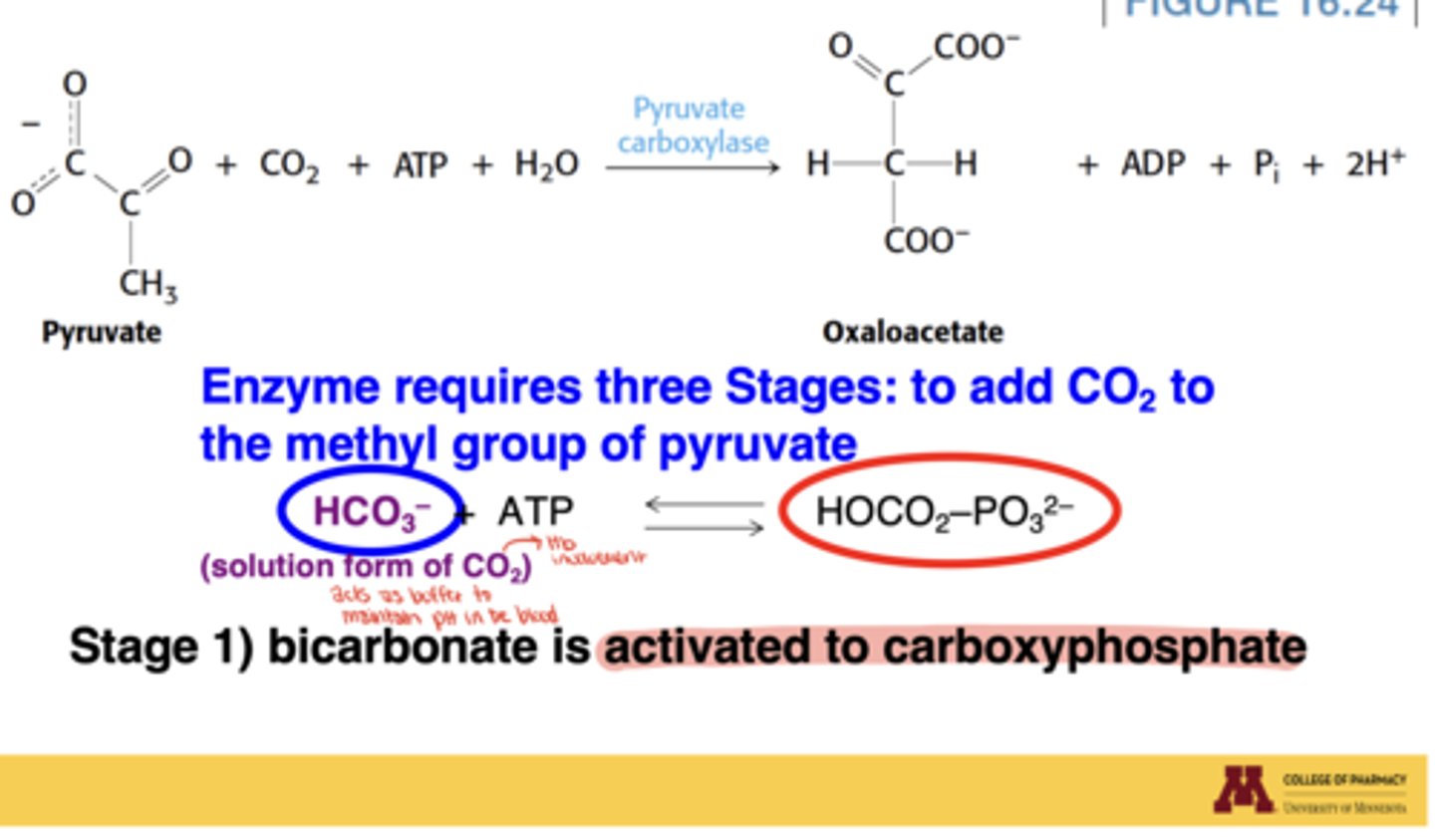
What is the first stage of the pyruvate carboxylase mechanism?
Bicarbonate is activated to carboxyphosphate
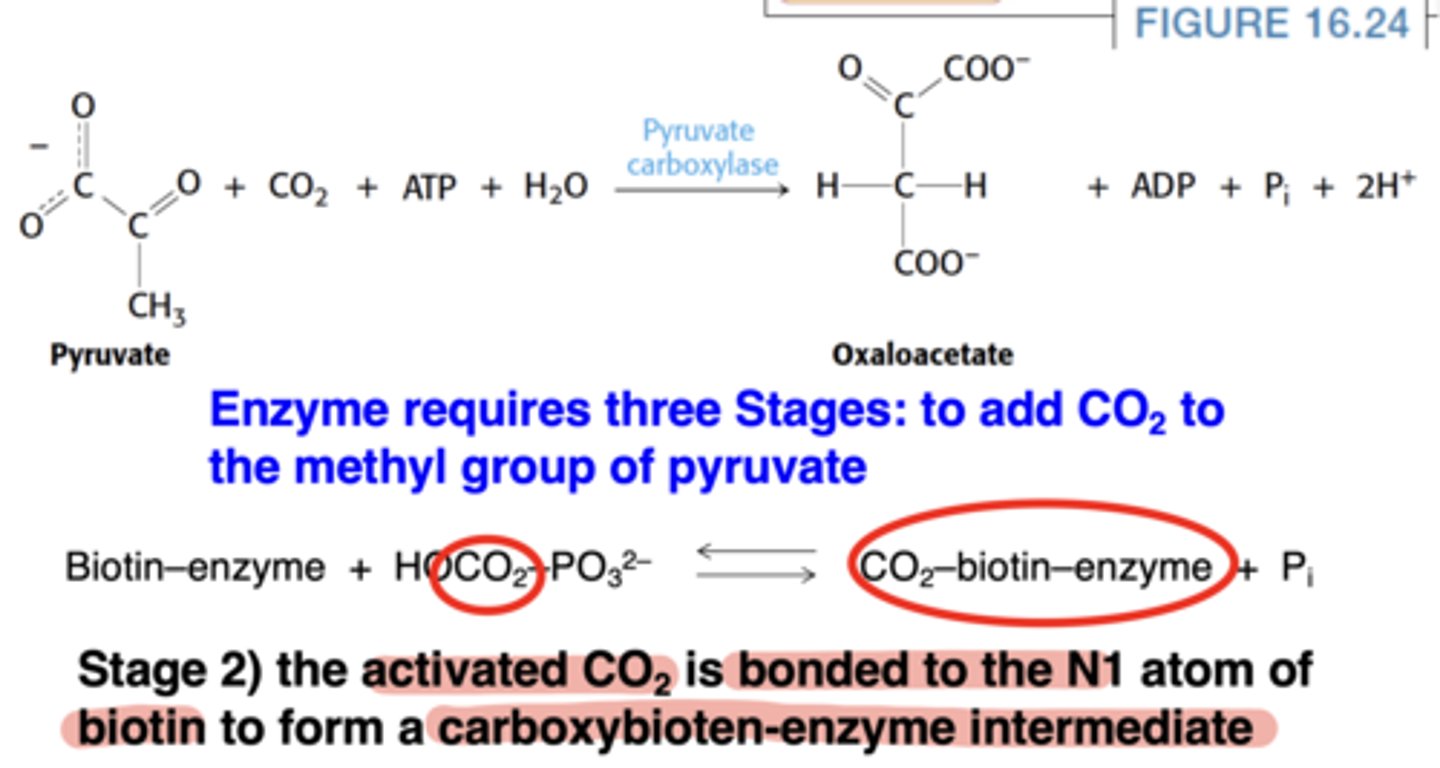
What is the second stage of the pyruvate carboxylase mechanism?
Activated CO₂ attaches to biotin (forming carboxybiotin-enzyme)
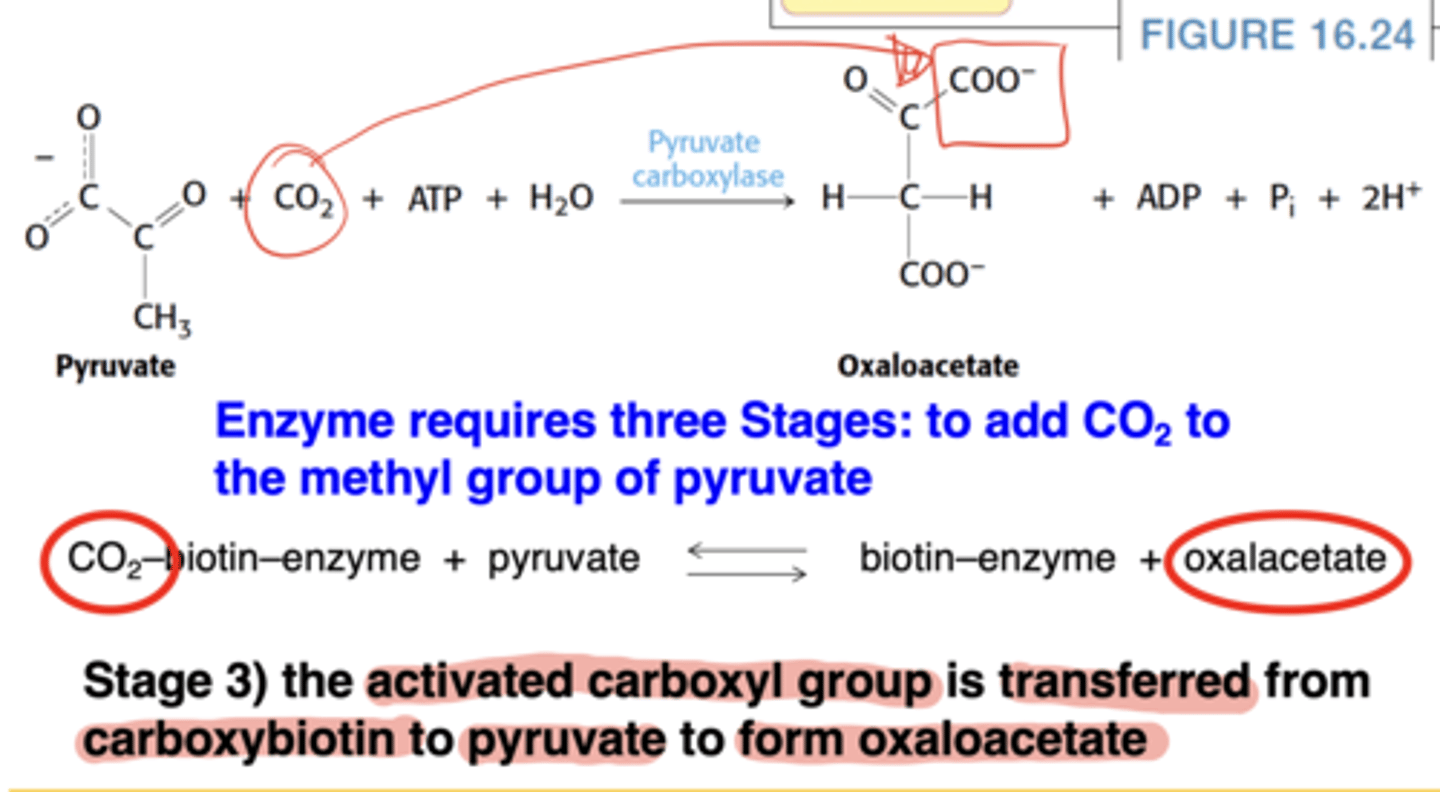
What is the third stage of the pyruvate carboxylase mechanism?
Carboxyl group transferred from biotin to pyruvate → oxaloacetate
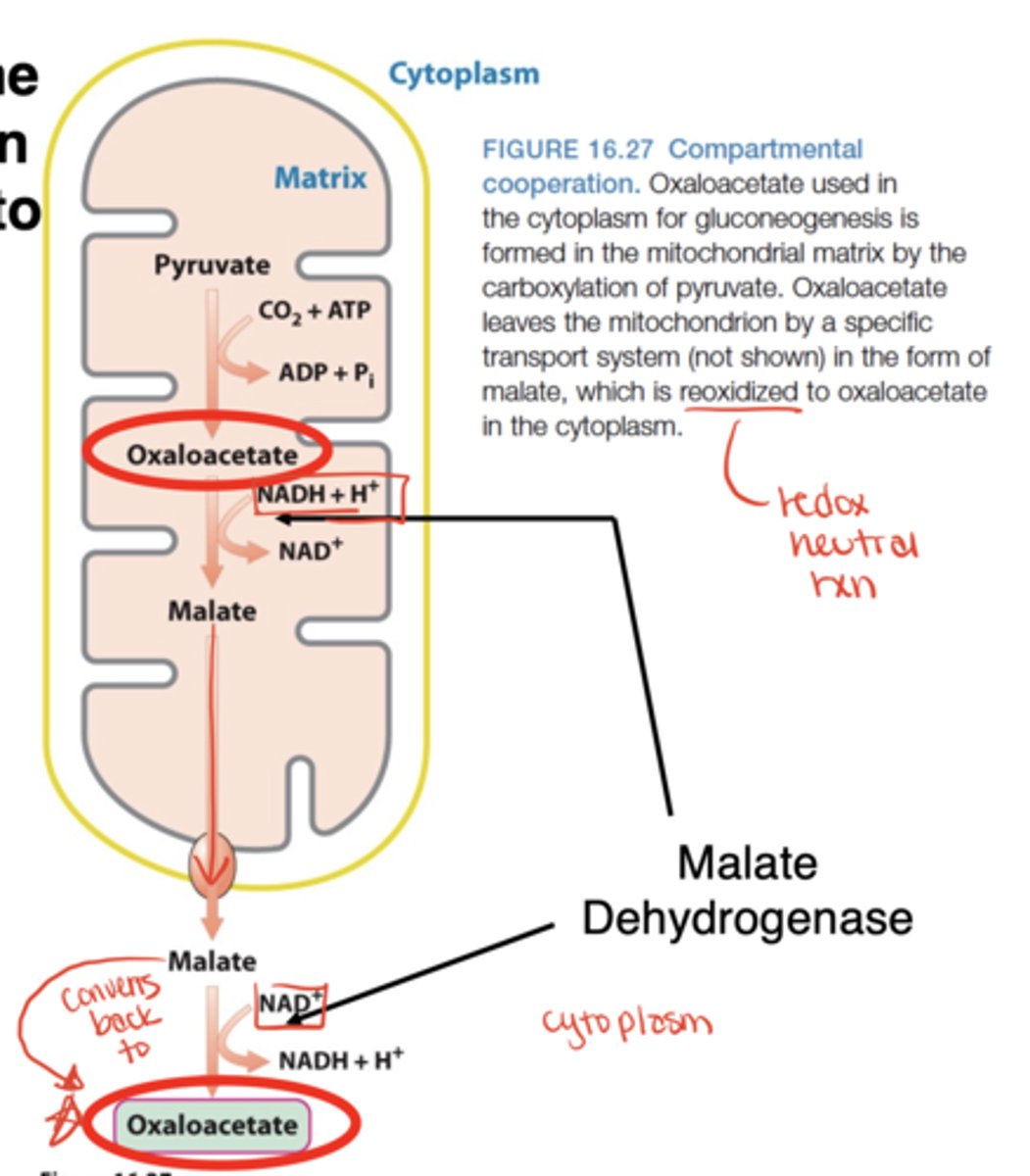
How is oxaloacetate moved from mitochondria to cytosol?
Converted to malate (by malate dehydrogenase), transported out, then reconverted to oxaloacetate
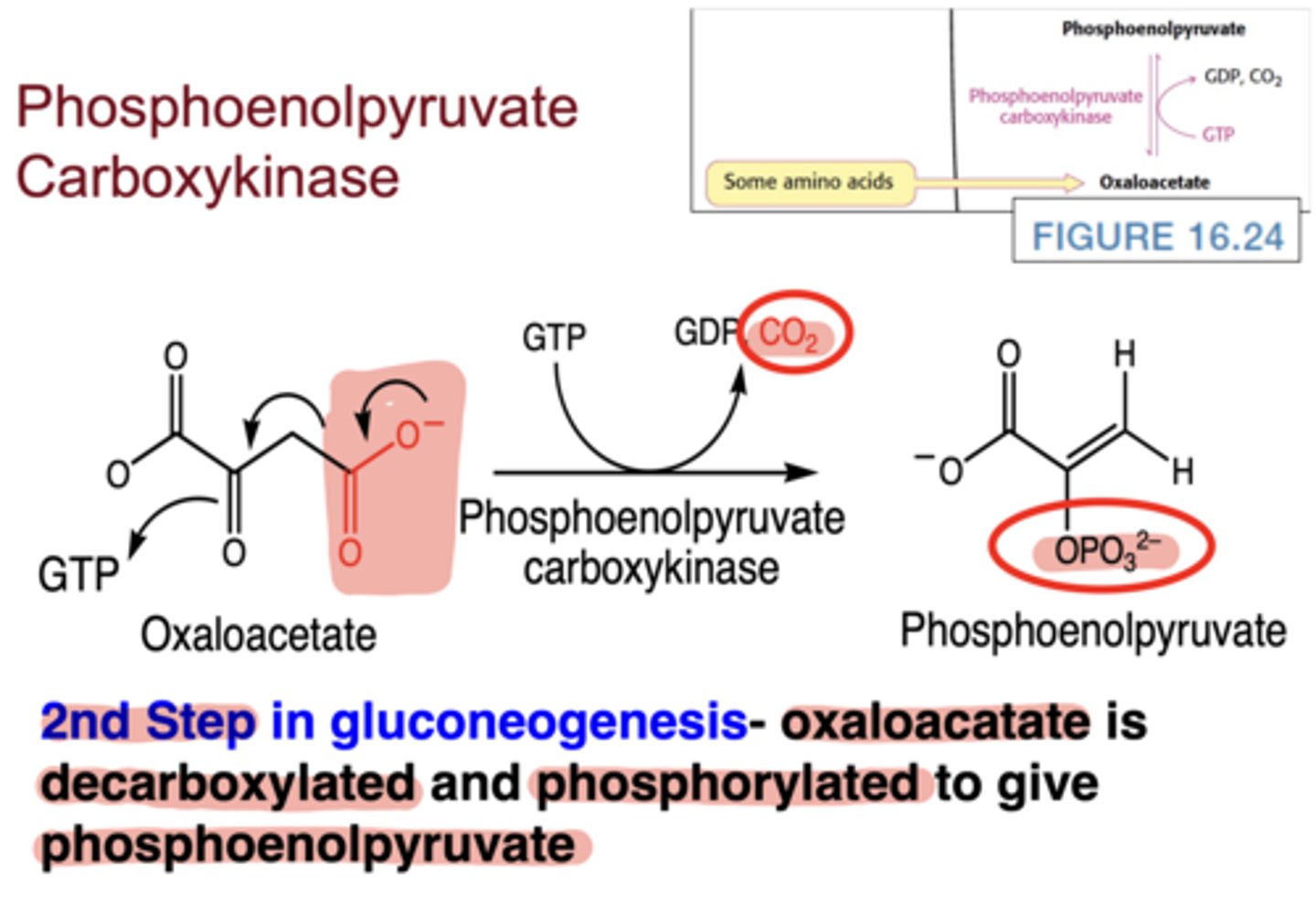
What reaction does PEP carboxykinase catalyze?
decarboxylation and phosphorylation of oxaloacetate → phosphoenolpyruvate (PEP), using GTP
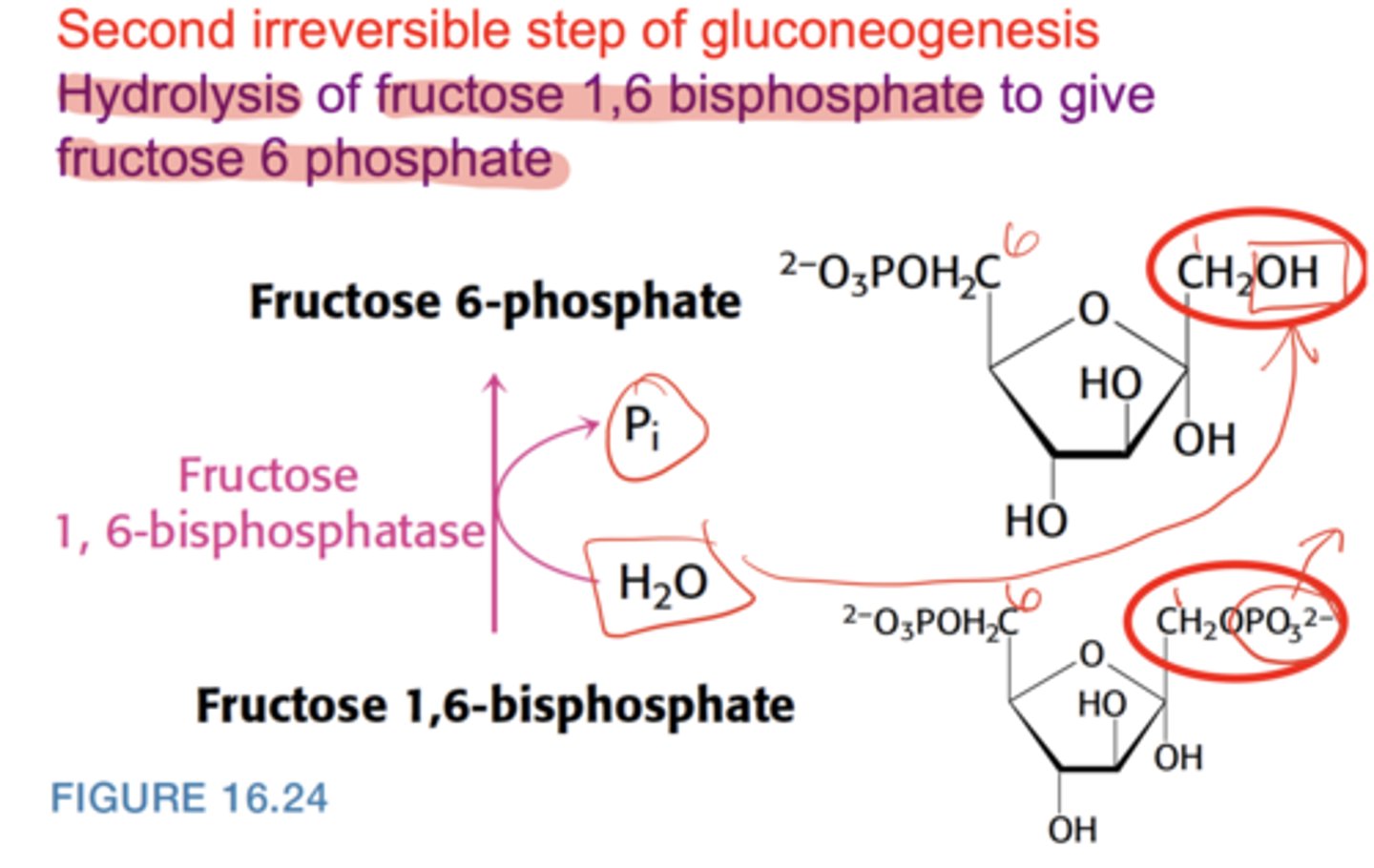
What is the second irreversible step of gluconeogenesis?
Hydrolysis of fructose-1,6-bisphosphate → fructose-6-phosphate
In most tissues, does gluconeogenesis end here?
Yes, only the liver (and kidney) continue to produce free glucose
What is the third irreversible step of gluconeogenesis?
Hydrolysis of glucose-6-phosphate → glucose
Where does this reaction occur?
In the endoplasmic reticulum, not the cytosol
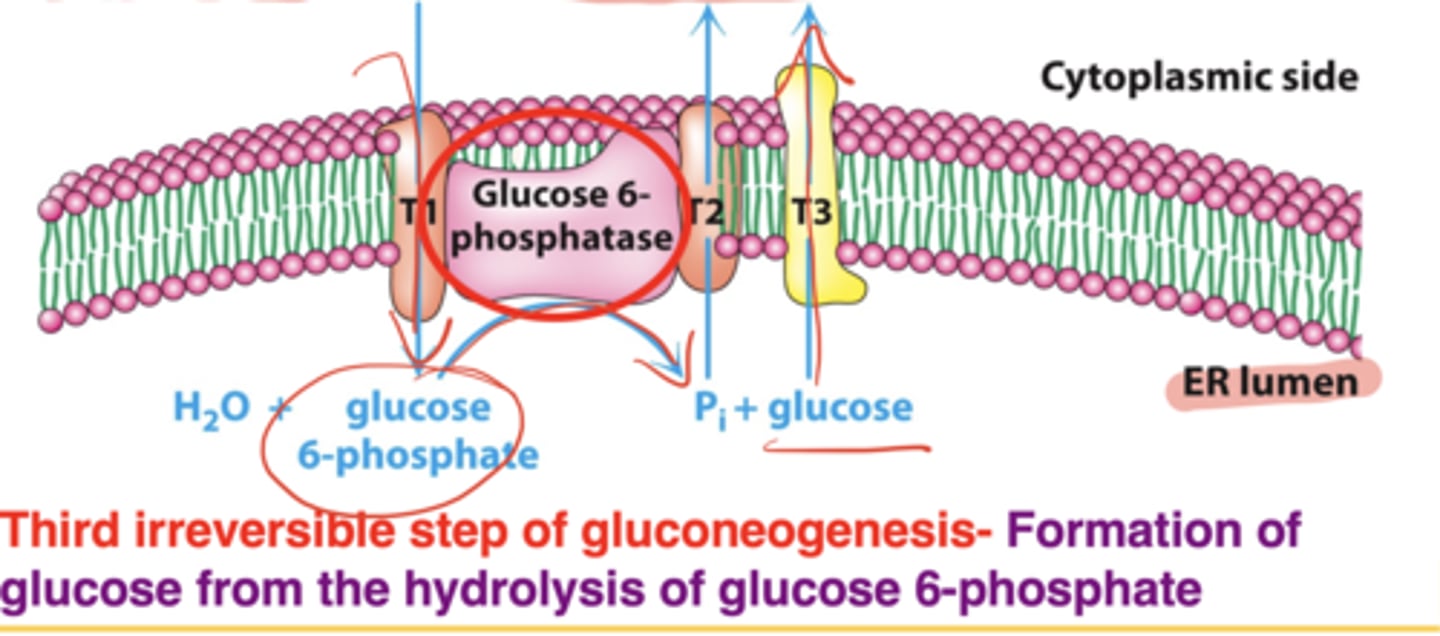
How many proteins are required, and what special cofactor is involved?
Five proteins; stabilizing protein requires calcium as a cofactor
Can glycolysis and gluconeogenesis both be highly active in the same cell?
No, only one pathway is highly active at a time
What drives glycolysis?
High glucose availability, ATP demand
What drives gluconeogenesis?
High ATP availability, abundance of precursors (lactate, alanine, glycerol)
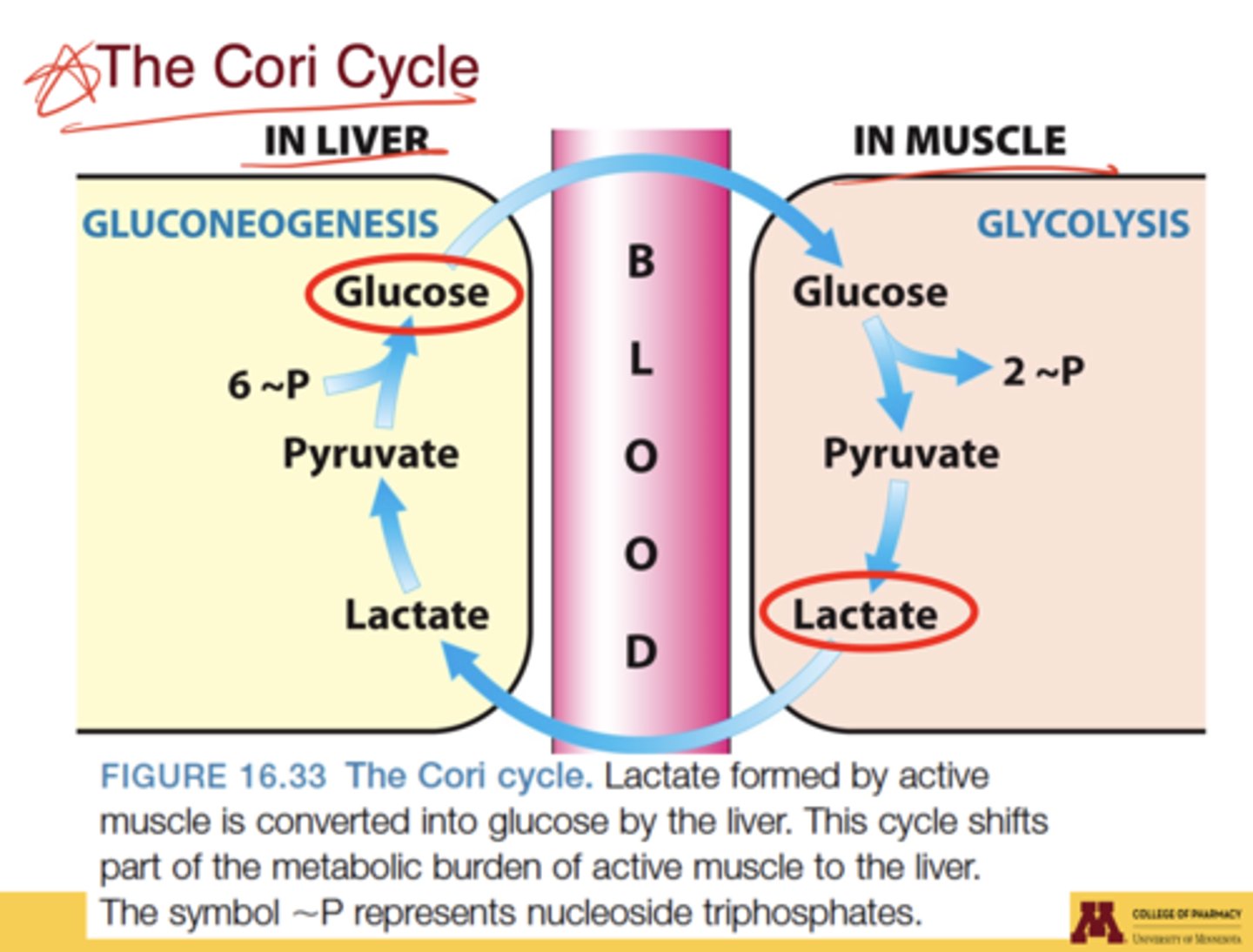
What is the Cori cycle?
metabolic cycle in which lactate from active muscle is transported to the liver, converted to glucose by gluconeogenesis, and returned to muscle via the blood
What is glycogen the readily mobilized storage form of?
Glucose
What are the two principal storage sites for glycogen?
Liver and skeletal muscle
What protein forms the yellow core of glycogen particles?
Glycogenin
What types of linkages are present in glycogen?
α(1→4) linkages: linear chains
α(1→6) linkages: branch points
Why is glycogen highly branched?
provides multiple nonreducing ends for rapid synthesis and breakdown
What percent glycogen by weight is stored in the liver vs muscle?
Liver: 10% by weight
Muscle: 2% by weight
Which tissue has more total glycogen content overall?
Muscle, due to its greater total mass
Which enzyme allows the liver to release free glucose into the blood?
Glucose-6-phosphatase
What is the primary role of liver glycogen?
to maintain blood-glucose levels for the whole body
What is the primary role of muscle glycogen?
To regulate energy needs of the muscle itself
What are the main regulators of glycogen metabolism?
Allosteric modulators (local energy state signals like ATP, AMP, glucose)
Hormones (insulin, glucagon, epinephrine, adjusting systemic needs)
Why does muscle glycogen stay in muscle instead of supporting blood glucose?
Muscle lacks glucose-6-phosphatase, so glucose-6-phosphate stays trapped in the cell
What is the Cori cycle's role between muscle and liver?
muscle exports lactate (from glycolysis), which the liver converts back to glucose via gluconeogenesis; this glucose re-enters blood and supplies muscle again
What is the key enzyme in glycogen breakdown?
Glycogen phosphorylase
What type of reaction does glycogen phosphorylase perform?
Phosphorolysis — bond cleavage by orthophosphate
Why is phosphorolysis energetically advantageous compared to hydrolysis?
It produces glucose-1-phosphate (G1P), which directly enters glycolysis without requiring ATP for phosphorylation
Why does G1P remain in muscle cells?
There are no transporters for G1P; it stays in the cell for local energy use
What cofactor does glycogen phosphorylase require?
Pyridoxal phosphate (PLP), derived from vitamin B6
How is PLP linked to the enzyme?
via a Schiff-base linkage to a lysine residue
What is PLP's role in glycogen breakdown?
Interacts with phosphate to facilitate phosphorolysis of glycogen
What is the quaternary structure of glycogen phosphorylase?
A dimer of 2 identical subunits (~97 kDa each, ~480 amino acids)
Where is the catalytic site located?
In a deep crevice formed by residues from both subunits
How does substrate binding affect the enzyme?
substrate binding narrows the crevice, excludes water, and allows accommodation of 4-5 glucose residues for efficient catalysis
What is the immediate product of glycogen phosphorylase activity?
Glucose-1-phosphate
How is this formed mechanistically?
A carbonium ion intermediate combines with orthophosphate to generate glucose-1-phosphate
Why is remodeling required during glycogen breakdown?
To remove branch points so phosphorylase can continue cleaving at nonreducing ends
What enzyme converts glucose-1-phosphate → glucose-6-phosphate?
Phosphoglucomutase
What are the two interconvertible forms of glycogen phosphorylase?
Phosphorylase a (usually active)
Phosphorylase b (usually inactive)
What are the two conformational states of phosphorylase?
R (relaxed) = more active
T (tense) = less active
How does tissue default state differ?
Liver: Default = a form (active, releasing glucose unless signaled otherwise)
Muscle: Default = b form (inactive until activated during contraction)
How does phosphorylase kinase regulate glycogen phosphorylase?
It phosphorylates phosphorylase b → phosphorylase a
How is phosphorylase kinase activated?
Initial activation: Ca²⁺ binding to δ subunit (calmodulin)
Maximal activation: Phosphorylation of α and γ subunits
Which hormone stimulates glycogen breakdown in muscle (and to a lesser extent liver)?
Epinephrine (adrenaline) from the adrenal medulla
Which hormone primarily triggers glycogen breakdown in the liver?
Glucagon from pancreatic α-cells (in response to low blood glucose)
What type of receptors mediate hormonal signals for glycogen breakdown?
G-protein coupled receptors (GPCRs)
What second messenger mediates glycogen breakdown signaling?
cAMP → activates protein kinase A (PKA)
How does the cascade amplify the signal?
Each step activates multiple downstream enzymes, producing a large glucose release response
How is glycogen breakdown signaling terminated?
GTPase (GTP → GDP).
Phosphodiesterase (cAMP → AMP).
Protein phosphatase (dephosphorylates enzymes)
What monomer is used in glycogen degradation?
Glucose-1-phosphate
What monomer is used in glycogen synthesis?
UDP-glucose
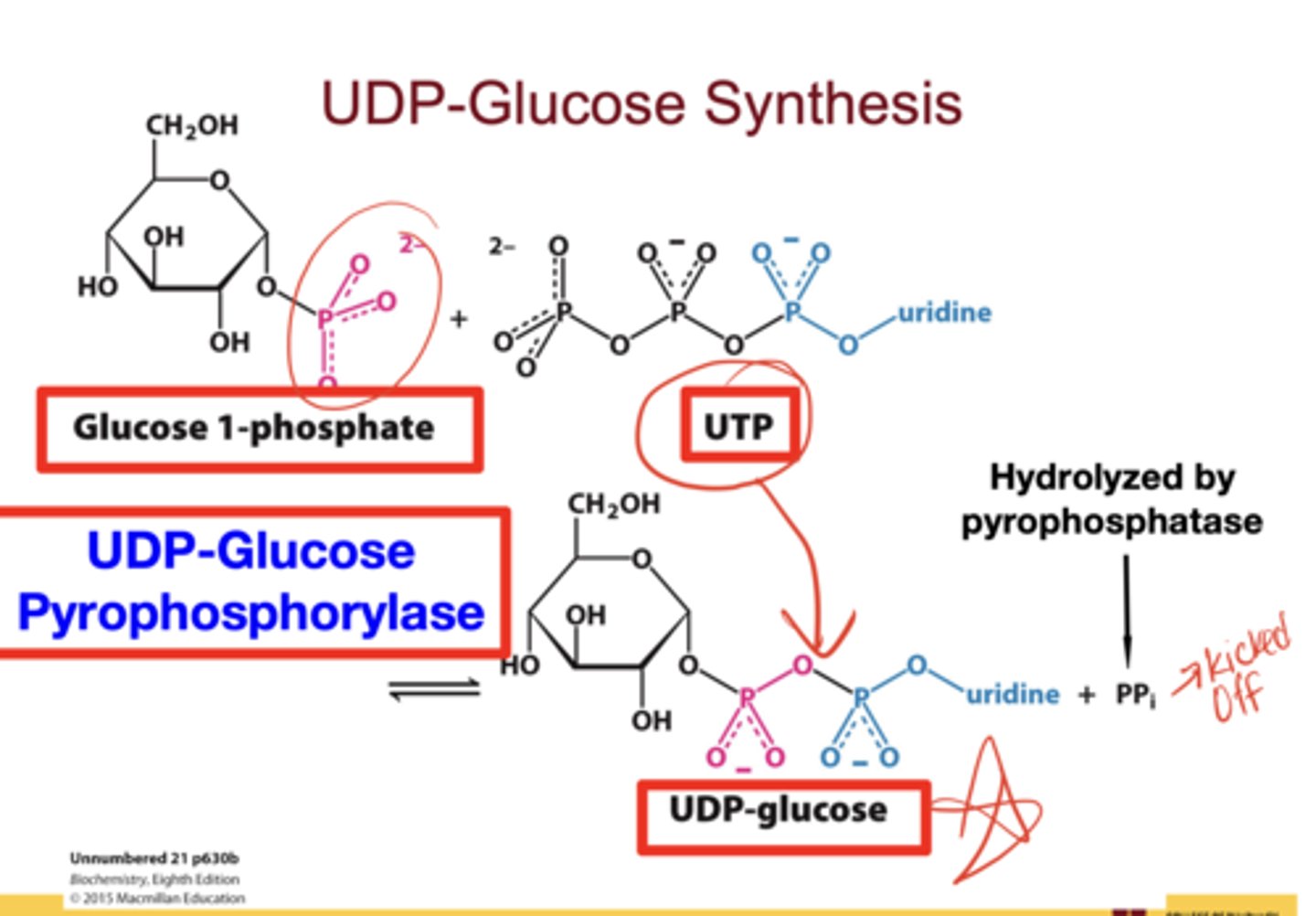
How is UDP-glucose synthesized?
Substrate: glucose-1-phosphate
Enzyme: UDP-glucose pyrophosphorylase
Reaction: G1P + UTP → UDP-glucose + PPi (hydrolyzed by pyrophosphatase, driving the reaction forward)
What is the key enzyme of glycogen synthesis?
Glycogen synthase
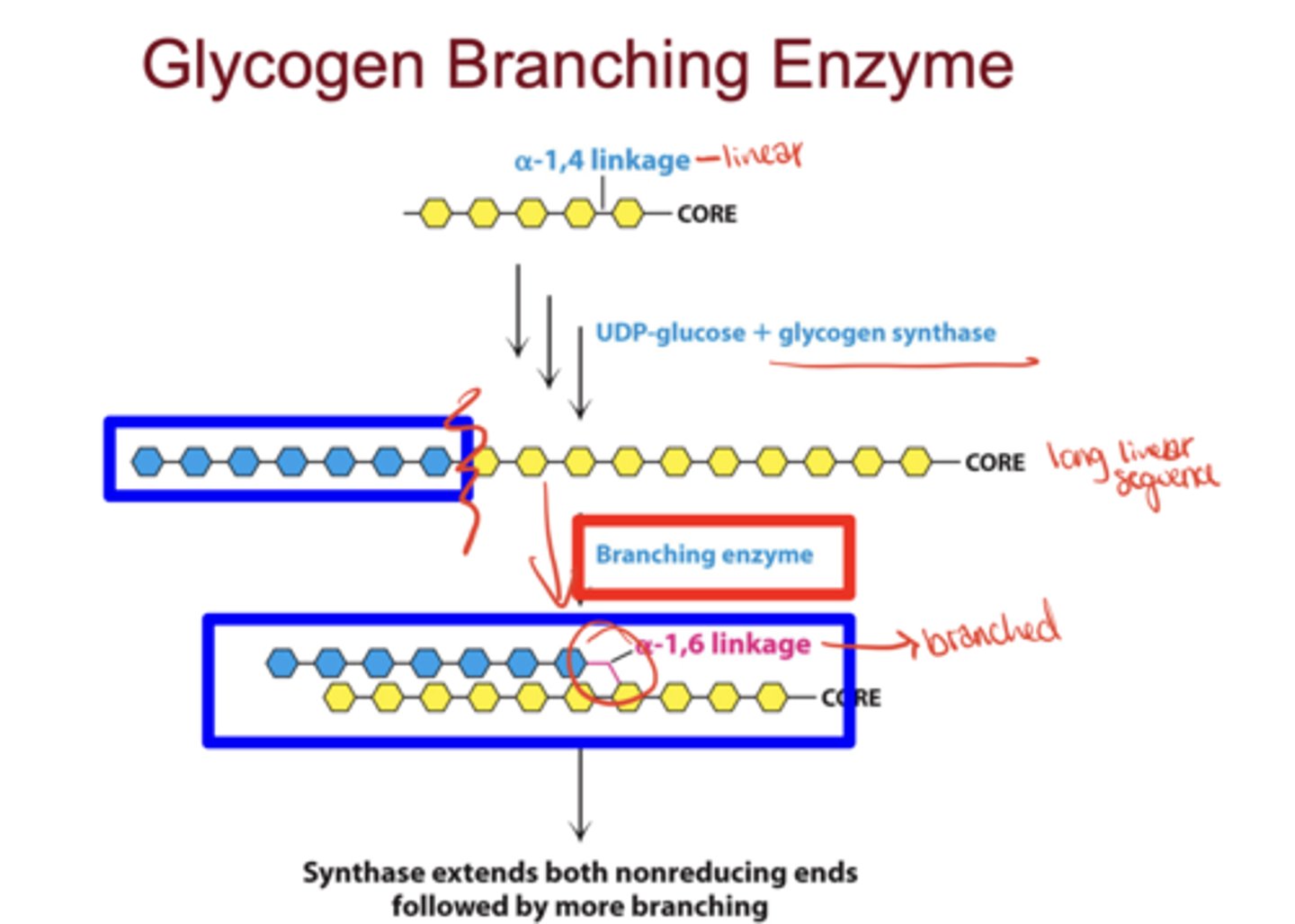
What reaction does glycogen synthase catalyze?
Transfers glucose from UDP-glucose to the nonreducing end of glycogen via an α(1→4) linkage (linear addition)
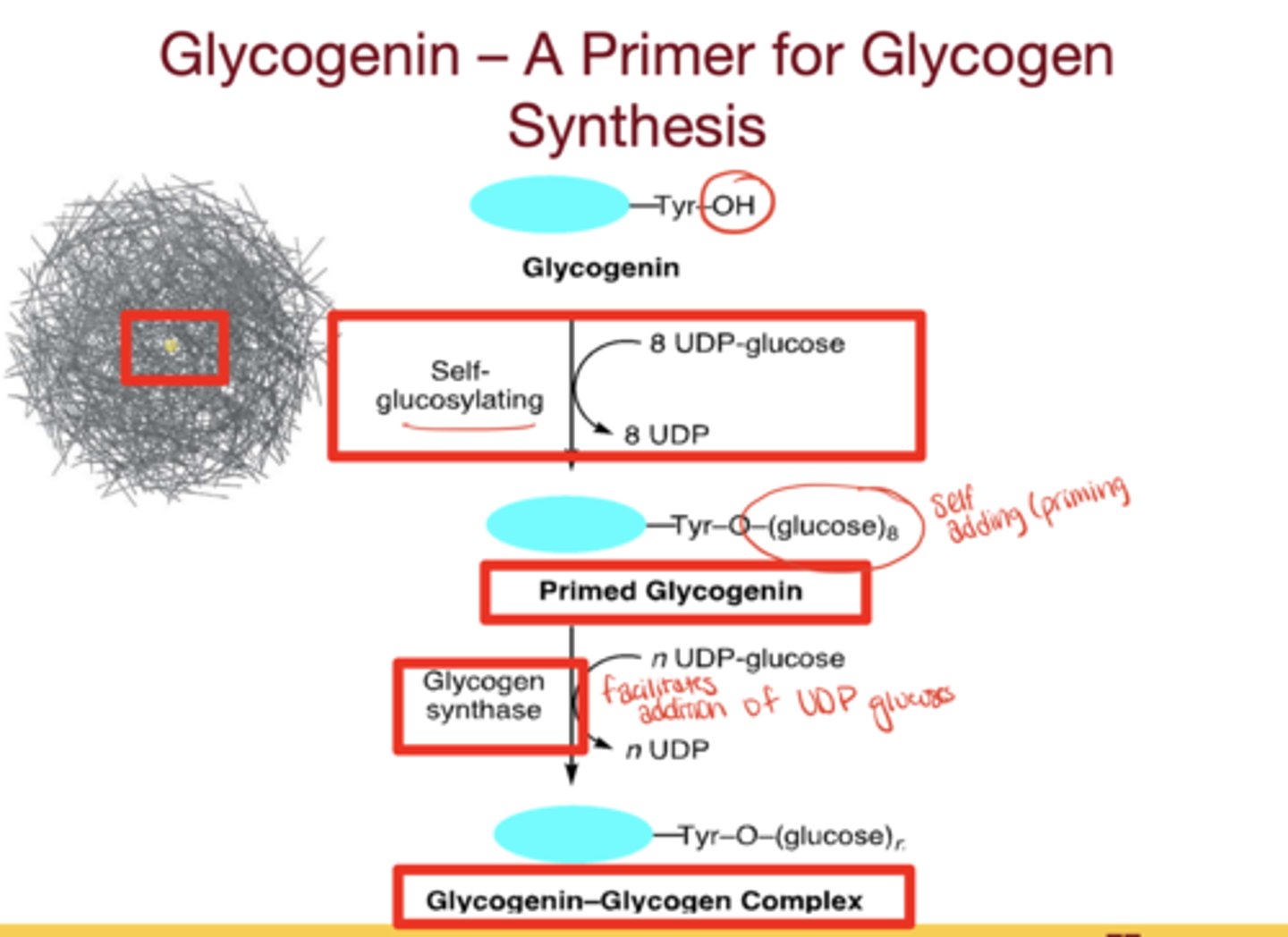
What is glycogenin?
protein primer for glycogen synthesis
What is the role of glycogenin?
- tyrosine residue with a hydroxyl group that initiates self-glycosylation with UDP-glucose
- ~8 glucose residues are attached, glycogen synthase recognizes the chain and elongates it
What type of linkage does glycogen synthase form?
α(1→4) linear linkages
What enzyme introduces branches in glycogen?
glycogen branching enzyme
What linkage does the branching enzyme create?
α(1→6) linkage (branch points)
Why must glycogen synthesis and breakdown be reciprocally regulated?
To prevent futile cycling; if both pathways were active simultaneously, ATP would be wasted
What enzyme is the key regulator of glycogen synthesis?
Glycogen synthase
What enzyme is the key regulator of glycogen breakdown?
Glycogen phosphorylase
In what two forms does glycogen synthase exist?
a form (active, dephosphorylated)
b form (inactive, phosphorylated)
How is glycogen synthase activated?
Dephosphorylation by protein phosphatase 1 (PP1)
How is glycogen synthase inhibited?
Phosphorylation by protein kinases
What is the function of PP1 in glycogen metabolism?
- activates glycogen synthase (by dephosphorylation).
- inactivates glycogen phosphorylase and phosphorylase kinase
How does PP1 coordinate glycogen metabolism?
Promotes glycogen synthesis and inhibits glycogen breakdown
How does insulin stimulate glycogen synthesis?
1. Activates PP1
2. Inactivates glycogen synthase kinase-3 (GSK-3), preventing further phosphorylation/inactivation of glycogen synthase
3. Increases glucose transport into muscle and adipose via GLUT4
What is the overall effect of insulin on glycogen metabolism?
Promotes glycogen synthesis, especially in the fed state
How does GSK-3 regulate glycogen synthase?
Phosphorylates and inactivates glycogen synthase (converts it to b form)
How does insulin signaling affect GSK-3?
Insulin signaling leads to GSK-3 inactivation, allowing glycogen synthase to remain active
How does glucose itself regulate glycogen metabolism in the liver?
- promotes dephosphorylation of glycogen phosphorylase (inactivating it)
- activates PP1 promoting glycogen synthase activity
During fasting, which hormones and signals change in the blood and tissue?
Blood: ↑ Glucagon, ↓ Insulin
Tissue: ↑ cAMP
What is the liver tissue response during fasting?
↑ Glycogen degradation, ↓ Glycogen synthesis
After a carbohydrate meal, which hormones/signals change in the blood and tissue?
Blood: ↓ Glucagon, ↑ Insulin, ↑ Glucose
Tissue: ↓ cAMP, ↑ Glucose
What is the liver tissue response after a carbohydrate meal?
↓ Glycogen degradation, ↑ Glycogen synthesis
During exercise or stress, which hormones/signals change in the blood and tissue?
Blood: ↑ Epinephrine
Tissue: ↑ cAMP, ↑ Ca²⁺–calmodulin
What is the liver tissue response during exercise/stress?
↑ Glycogen degradation, ↓ Glycogen synthesis
During fasting (rest), which regulator changes in the blood?
↓ Insulin (glucagon has little effect)
What is the muscle tissue response during fasting?
↓ Glycogen synthesis, ↓ Glucose transport
After a carbohydrate meal, which regulator changes in the blood?
↑ Insulin
What is the muscle tissue response after a carbohydrate meal?
↑ Glycogen synthesis, ↑ Glucose transport
During exercise, which regulators/signals change in the blood and tissue?
Blood: ↑ Epinephrine
Tissue: ↑ AMP, ↑ Ca²⁺–calmodulin, ↑ cAMP
What is the muscle tissue response during exercise?
↓ Glycogen synthesis, ↑ Glycogen degradation, ↑ Glycolysis