Electrons and Ionisation
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
An atomic orbital is a ...... around the nucleus that can hold up to .... electrons, with opposite spins.
region, two
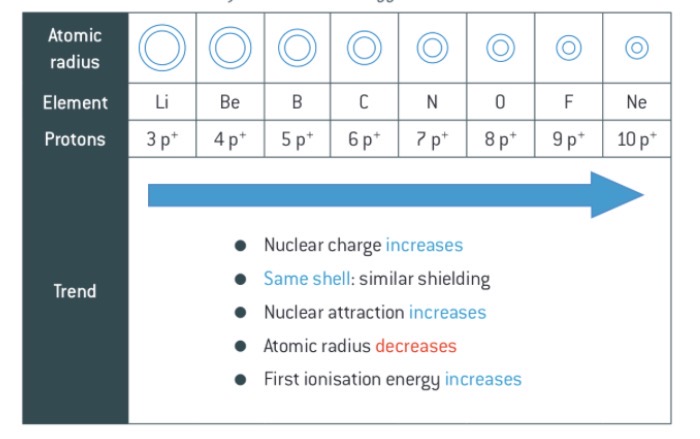
Can you explain the trend first ionisation energy across a period using these key words: nuclear charge, shells and shielding, nuclear attraction, atomic radius, and first ionisation energy. Word this like an exam response.
First ionisation energy is the energy required to remove one ........ from each atom in one mole of ...... atoms of an element to form
one mole of gaseous 1+ ions.
Electron, gaseous
How do you tell which element is which using the successive ionisation energies?
Write the electronic configuration then use the (Use Analia Sanchez's Video for support).