General Chemistry 2: Properties of Solutions
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Composed of a solute and a solvent.
Solution
A mixture where all parts have the same properties and chemical composition.
Homogeneous Mixture
The substance that is dissolved in the solution.
Solute
The substance in which the solute dissolves.
Solvent
A substance that dissolves in another substance.
Soluble
A substance that does not dissolve in another substance.
Insoluble
Liquids which are completely soluble in each other.
Miscible
Liquids that do not mix.
Immiscible
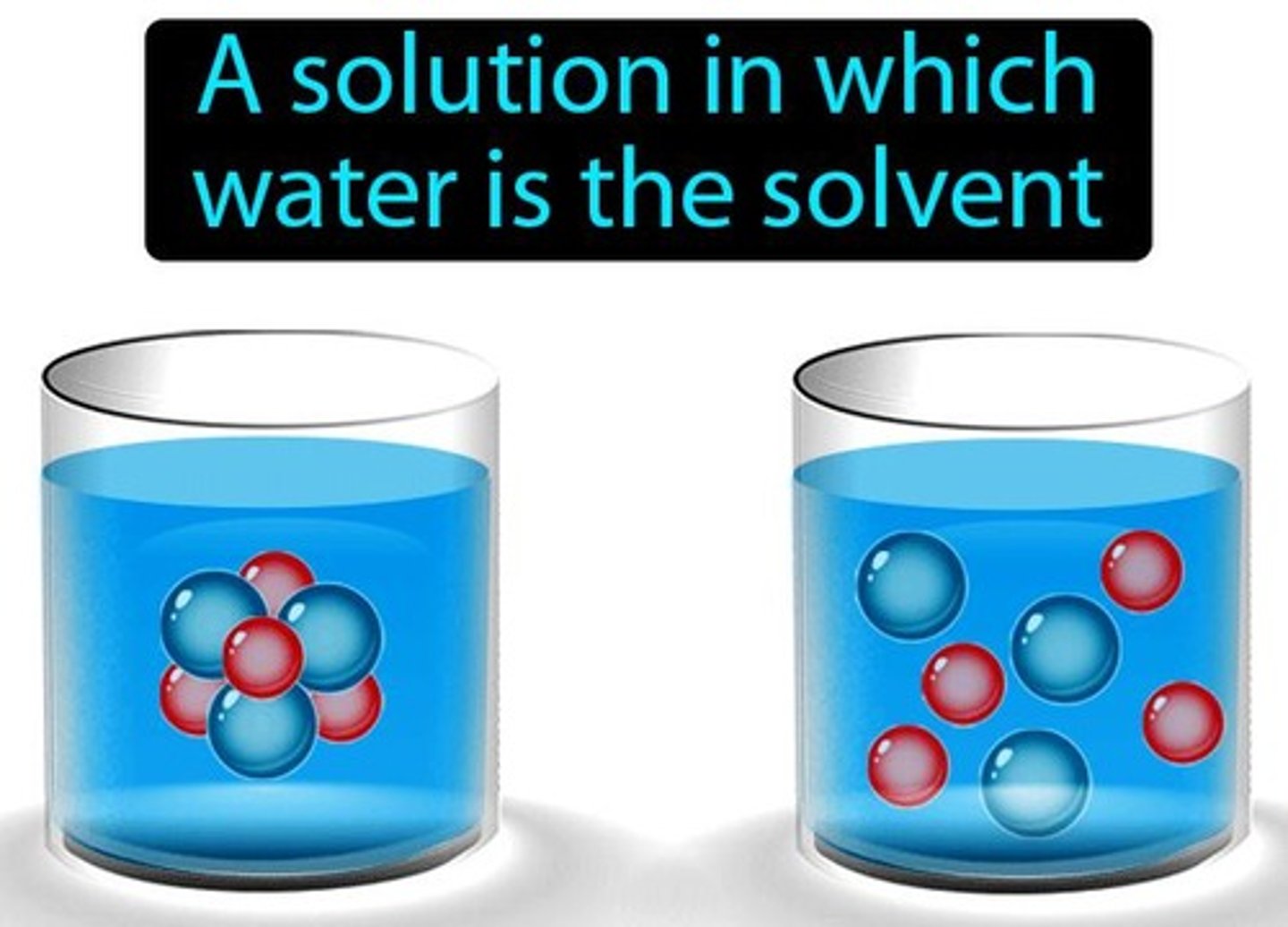
A solution in which the solvent is water.
Aqueous Solution
A substance that dissolves in water to form a solution that conducts electric current.
Electrolyte Solution
A substance that dissolves in water to give a solution that does not conduct electric current.
Non-Electrolyte
Gas in gas, such as air.
Gaseous Solution
Gas in liquid or liquid in liquid, such as soda water or antifreeze.
Liquid Solutions
Solid in liquid, liquid in solid, or solid in solid, such as dental amalgam or steel.
Solid Solutions
A solution that contains the maximum amount of solute that can dissolve at a given temperature and pressure.
Saturated Solution
A solution that contains less solute than a saturated solution.
Unsaturated Solution
A solution that contains more solute than a saturated solution and is very unstable.
Supersaturated Solution

The state where the rate of dissolving is equal to the rate of crystallization.
Dynamic Equilibrium
The higher the temperature of the solvent, the greater the solubility of the solute, except for gaseous solutes.
Temperature
This does not affect the solubility of most solutes in a solvent, except for gaseous solutes.
Pressure
Solutions can be described qualitatively or quantitatively based on the amount of solute relative to a given amount of solvent.
Concentration of Solute
Contains a relatively small amount of solute.
Dilute Solution
Contains a relatively large amount of solute.
Concentrated Solution
A unit for expressing very dilute concentrations, commonly used to express pollutants in air and water.
Parts Per Million (PPM)
The number of moles of solute per liter of solution.
Molarity (M)
The number of moles of solute per kilogram of solvent.
Molality (m)
The amount of the component of the solution expressed in moles per mol of solution.
Mole Fraction (Xi)
The mass of a solute to the mass of the solution multiplied by 100.
Percent by Mass
The volume (mL) of solute (liquid) to volume (mL) of the solution multiplied by 100.
Percent by Volume