VSEPR ONLY Chem Chapter 6
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
VSEPR theory
Valence-shell electron-pair repulsion theory; because electron pairs repel, molecules adjust their shapes so that valence electron pairs are as far apart as possible
Linear - Atoms bonded to central atom
2
Linear - Lone pairs of electrons
0
Linear - Type of molecule
AB₂
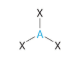
Trigonal-planar - Atoms bonded to central atom
3
Trigonal-planar - Lone pairs of electrons
0
Trigonal-planar - Type of molecule
AB₃
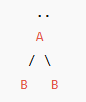
Bent or Angular (1 lone pair) - Atoms bonded to central atom
2
Bent or Angular (1 lone pair) - Lone pairs of electrons
1
Bent or Angular (1 lone pair) - Type of molecule
AB₂E
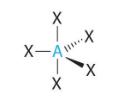
Tetrahedral - Atoms bonded to central atom
4
Tetrahedral - Lone pairs of electrons
0
Tetrahedral - Type of molecule
AB₄
Trigonal-pyramidal - Atoms bonded to central atom
3
Trigonal-pyramidal - Lone pairs of electrons
1
Trigonal-pyramidal - Type of molecule
AB₃E
Bent or Angular (2 lone pairs) - Atoms bonded to central atom
2
Bent or Angular (2 lone pairs) - Lone pairs of electrons
2
Bent or Angular (2 lone pairs) - Type of molecule
AB₂E₂
Trigonal-bipyramidal - Atoms bonded to central atom
5
Trigonal-bipyramidal - Lone pairs of electrons
0
Trigonal-bipyramidal - Type of molecule
AB₅
Octahedral - Atoms bonded to central atom
6
Octahedral - Lone pairs of electrons
0
Octahedral - Type of molecule
AB₆.
Linear VSEPR Diagram
180 degrees
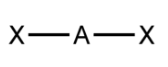
Trigonal-planar VSEPR Diagram
120 degrees
Bent or angular (1 lone pair) VSEPR Diagram
120 degrees
Tetrahedral VSEPR Diagram
109.5 degrees
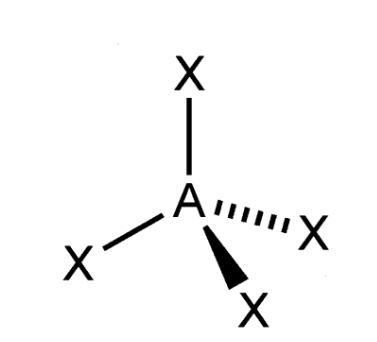
trigonal-pyramidal VSEPR Diagram
107 degrees
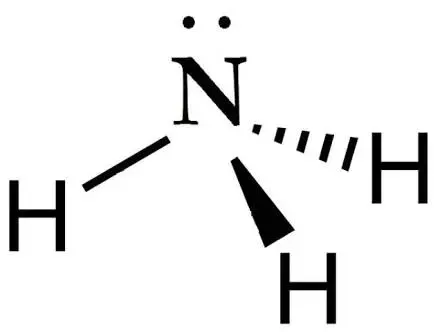
Octahedral VSEPR Diagram
90 degrees
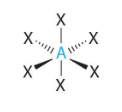
Bent or angular (2 lone paris) VSEPR Diagram
104.5 degrees
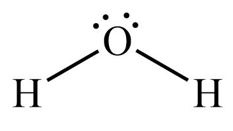
Triognal Bipyramidal VSEPR Diagram
90 and 120 degrees