OCR A LEVELS - organic chemistry
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
65 Terms
how many bonds can a carbon form
4
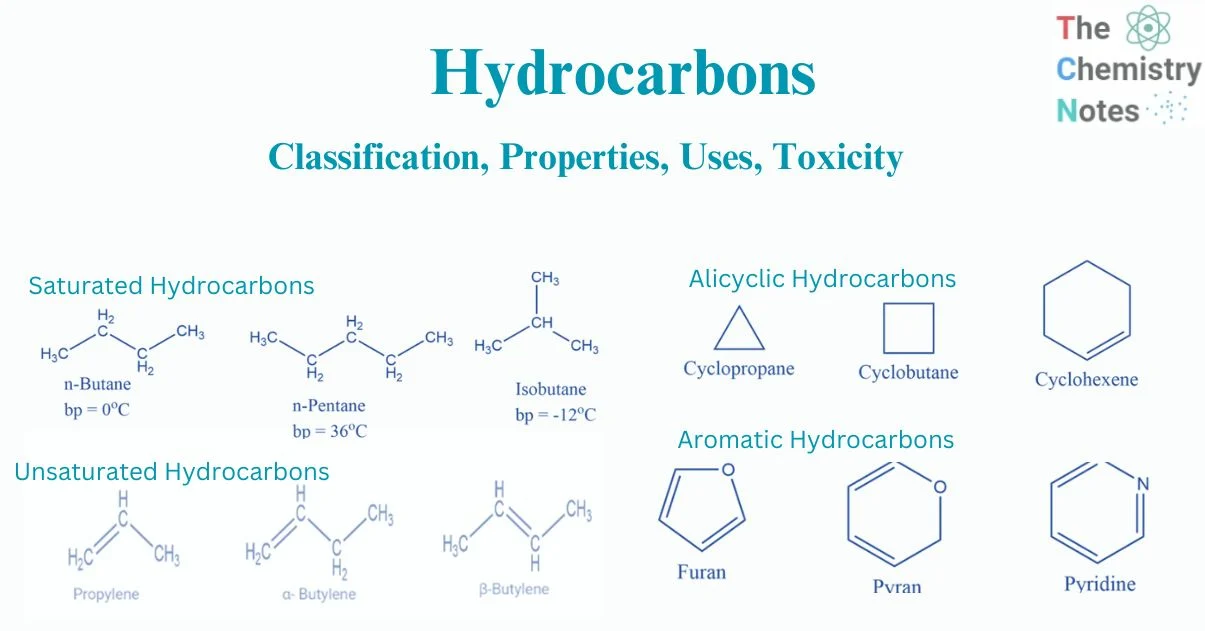
define hydrocarbon
compound consisting of carbon and hydrogen
define saturated hydrocarbons
single carbon–carbon bonds only
define unsaturated hydrocarbons
the presence of multiple carbon–carbon bonds, including C=C, C C / and aromatic rings
define homologous series
a series of organic compounds having the ssame functional group but with each successive members differing by CH2
define functional group
a specific group of atoms that are within organic molecules and are responsible for characteristic properties and reactivities of those molecules
define aliphatic
a compound containing carbon and hydrogen joined together in straight chains, branched chains or non-aromatic rings
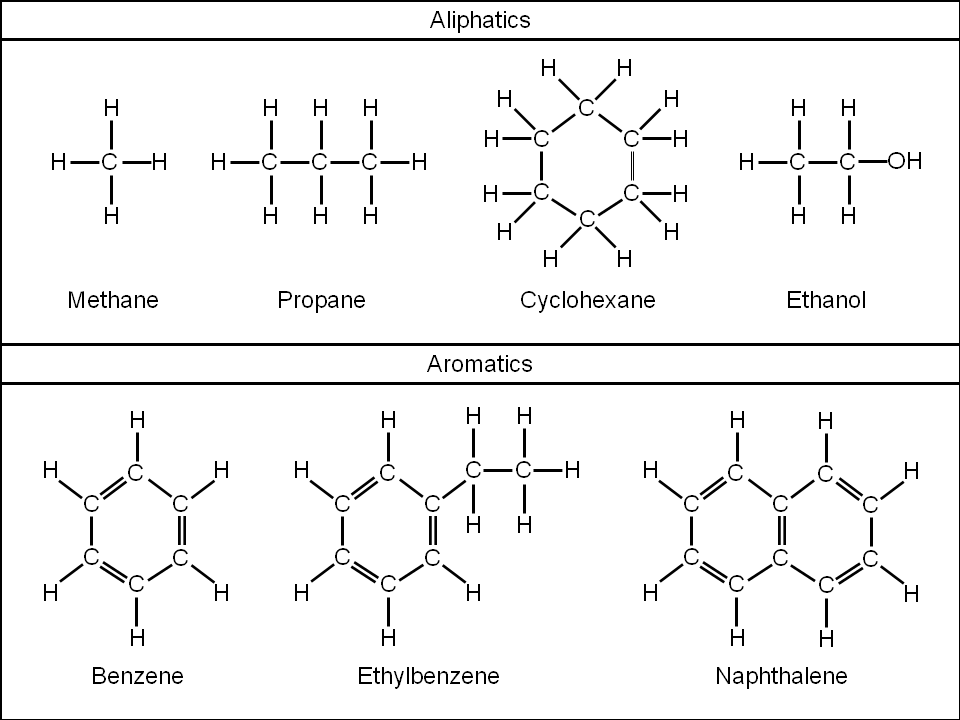
define alicyclic
an aliphatic compound arranged in non-aromatic rings with or without side chains
define aromatic
a compound containing a benzene ring
what are the 3 homologous series of aliphatic hydrocarbons
alkanes
alkenes
alkynes
name first 10 alkanes
methane = CH4
ethane = C2H6
propane = C3H8
butane = C4H10
pentane = C5H12
hexane = C6H14
heptane = C7H16
octane = C8H18
nonane = C9H20
decane = C10H22
name first 10 alkyl groups (side chains)
methyl = CH3
ethyl = C2H5
propyl = C3H7
butyl = C4H9
pentyl = C5H11
hexyl = C6H13
heptyl = C7H15
octyl = C8H17
nonyl = C9H19
decyl = C10H21
R can be used to represent alkyl group
general formula: CnH2n+1
how to name alkanes
identify longest chain of carbon atoms
identify any side chains attached to the parent chain
add numbers before any side chains to show position of the alkyl group
general formula for alkanes
CnH2n+2 —→ contains a single C-C bond
naming alicyclic alkanes
identify continuous chain of carbon atoms
add the prefix cyclo infront of alkane
naming alkenes
identify the longest continuous chain of carbon atoms aka stem
identify where the double bond is
combine the stem with ene and the position of the double bond of the compound
general formula for alkenes
CnH2n —→ contains a double bond
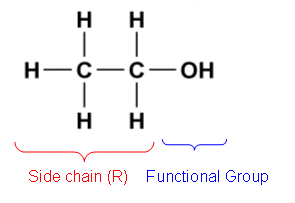
alcohol
functional group: -OH
prefix: hydrox-
suffix: ol
general formula: CnH2n+1OH
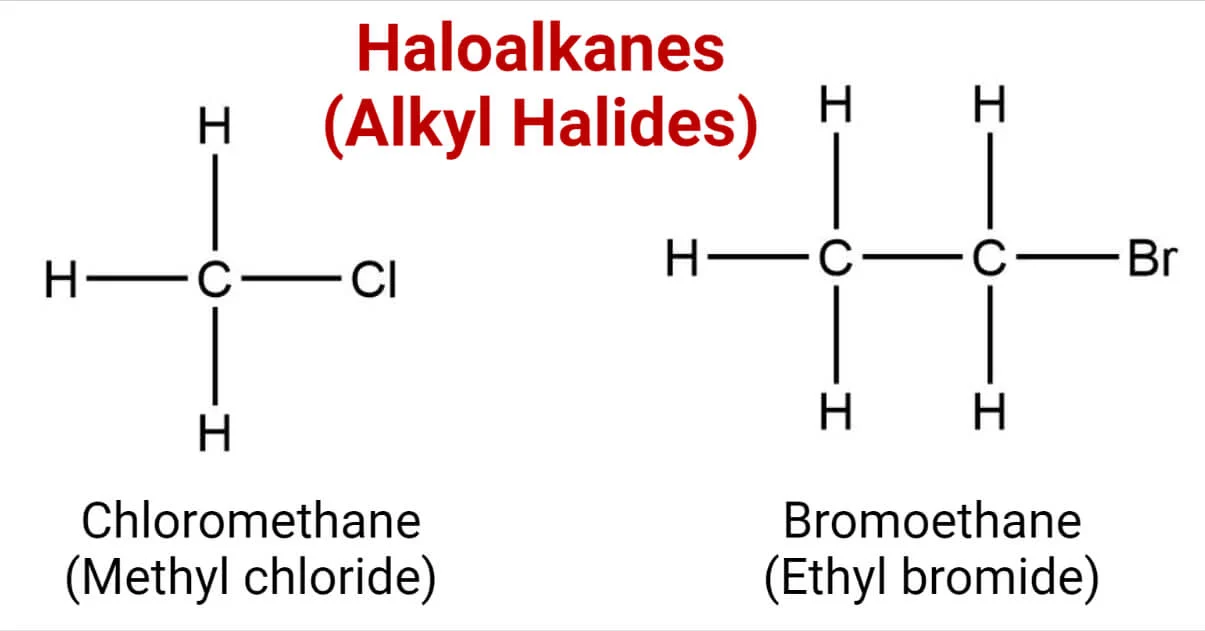
haloalkanes
functional group: -Cl -Br -I
prefix: chloro bromo iodo
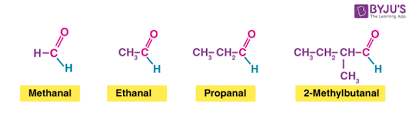
aldehyde
functional group: -CHO c double bonds to O
suffix: -al
always on position 1
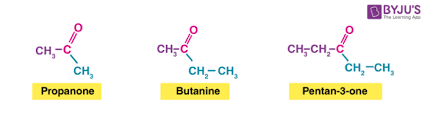
ketone
functional group: -C(CO)C- c double bonds to o
suffix: -one
general formula: CnH2nO
carboxylic acid
functional group: -COOH c double bonds to o
suffix: -oic acid
general formula:CnH2nO2
naming alcohol
identify functional group and suffix
identify longest chain of carbon atoms
identify which carbon atom the functiona groups on
combine the suffix and stem and the position the functional group is on togather.
define general formula
the simplest algebraic formula of a member of a homologous series
e.g. for an alkane: CnH2n+2
define structural formula
the minimal detail that shows the arrangement of atoms in a molecule
e.g. for butane: CH3CH2CH2CH3 or CH3(CH2) 2CH3
carboxyl group: COOH
ester group: COO
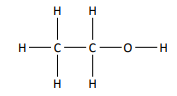
define displayed formula
the relative positioning of atoms and the bonds between them
e.g. for ethanol:
define skeletal formula
the simplified organic formula, shown by removing hydrogen atoms from alkyl chains, leaving just a carbon skeleton and associated functional groups
e.g. for butan-2-ol:
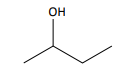
displayed and skeletal for cyclohexane
for hexene it has one c=c bond and another line inside of hexagon
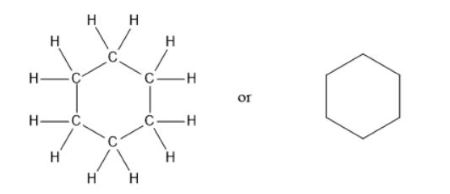
bezene ring
C6H6
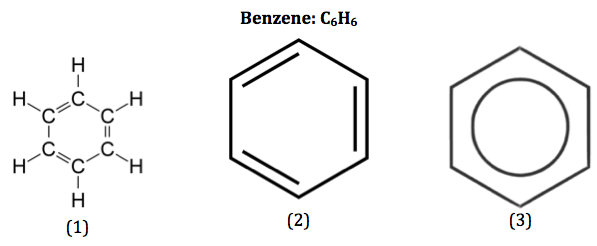
define structural isomers
compounds with the same molecular formula but different structural formulae
isomers with the same molecular formula but different structural isomers
aldehydes and ketones = same molecular formula but different functional group
e.g. C3H6O
aldehyde: propanal
ketone: propanone
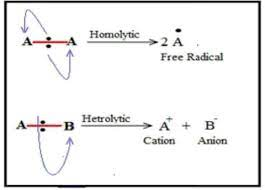
define homolytic fission
in terms of each bonding atom receiving one electron from the bonded pair, forming two radicals
each atom has a single unpaired electron
so a radical is formed
e.g H3C-CH3 —> H3C. + .CH3
ethane
define heterlytic fission
in terms of one bonding atom receiving both electrons from the bonded pair
the atom that takes both electrons becomes negative ion
atom that doesnt take electrons is positive ions
e.g H3C-Cl —> H3C+Cl-
chloromethane
uses a curly arrow
bond fission occurs in
a covalent bond in alkanes
define radical
a species with an unpaired electron
‘dots’ to represent species that are radicals in mechanisms
Radical mechanisms will be represented by a sequence of equations.
Dots, •, are required in all instances where there is a single unpaired electron (e.g. Cl• and CH3•). Dots are not required for species that are diradicals (e.g. O)
define curly arrow
movement of an electron pair, showing either heterolytic fission or formation of a covalent bond
Curly arrows should start from a bond, a lone pair of electrons or a negative charge.
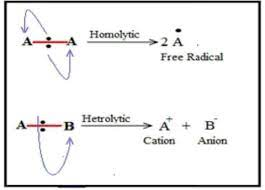
half headed arrows
is used for homolytic fission and represents the movement of a single unpaired electron mechanism involving radicals.
3 types of structural isomer
chain isomers
positional isomers
functional group isomers
define chain isomer
shown as skeletal formula
these isomers have similar chemical properties but their physical properties e.g. boiling point will be different as the shape of the molecule is different
define positional isomer
skeletal and functional group remains the same but the functional group is attached to a different carbon atoms
different physical and chemical properties
define functional group isomers
same atoms arranged in different functional group
so different physical and chemical properties
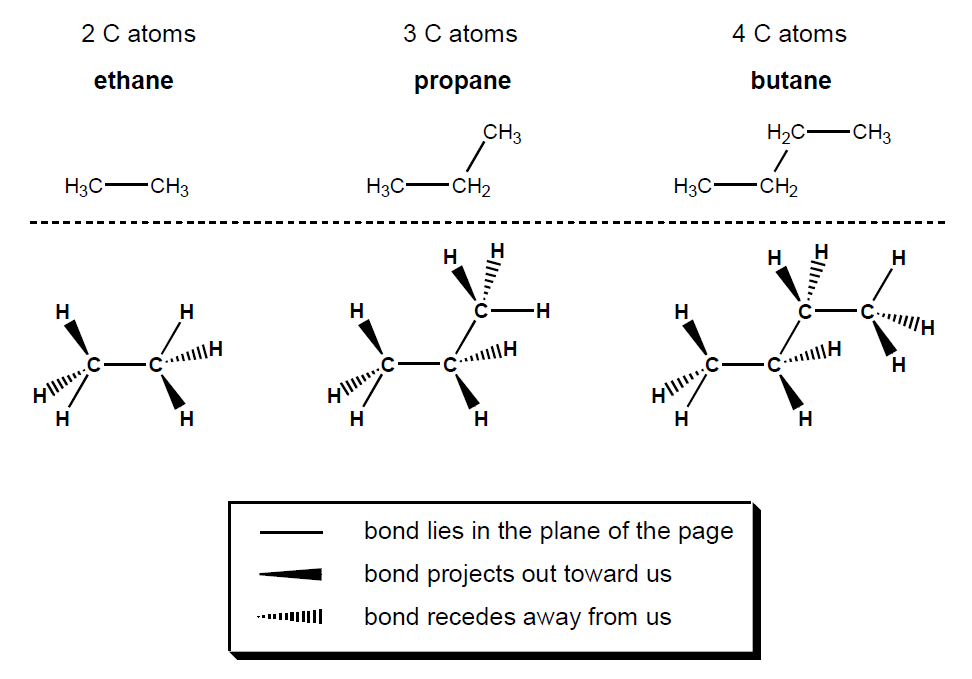
shape and bond angle of all alkanes
they are all tetrahedral shape around each carbon
these bonded pairs all repel equally
bond angle is 109.5
alkanes are held by what type of bonds
alkanes consists of covalent bonds
between the molecules there are induced dipole dipole interaction aka London Forces
the longer the carbon chain (straight chain) in alkane means
stronger the induced dipole dipole interaction
this is because theres more surface contact and more electrons to interact with
more energy required to overcome to overcome the induced dipole dipole interaction - high BP
branched carbon chain alkane means
theres a lower BP than straight chained isomer
they cant pack closley togather
they have a smaller molecular surface area
this means induced dipole dipole interactions are reduced
complete combustion reaction: ALKANES
formula: alkane + oxygen —> carbon dioxide + water
combustion happens between gases, so liquid alkanes must be vapourised first. smaller alkanes turns into gases EASILY (more volatile) so burns easily
larger alkanes releases ALOT of energy because they have more bonds to react too
due to so much energy being released alkanes are excellent FUELS.
incomplete combustion reaction: ALKANES
formula: alkane + oxygen —> carbon monoxide + water
there is only a limited amount of oxygen in this reaction
carbon monoxide is poisonous
carbon monoxide binds better with haemoglobin in the bloodstream than oxygen, this means less oxygen can be carried around the body —- OXYGEN DEPRIVATION.
reaction of alkanes with halogens
under the condition of UV light, the initial energy is given to start the reaction
e.g. chlorine + methane —> chloromethane
overall reaction equation: Cl2 + CH4 —> CH3Cl + HCl
hydrogen atom is substituated by chlorine or bromine = free radical substituation reaction.
the 3 steps in the reaction mechanism for bromination/chlorination of alkane
Initation
Propagation
Termination
Initation of chlorination of methane
Initation - free radicals are produced
under UV light enough energy is provided to break the Cl-Cl OR Br-Br bond = photodissociation
the bond splits equally and each atom gets to keep one electron - homolytic fission
the atom becomes a highly reactive free radical because of its unpaired electrons
e.g. Cl2 —> 2Cl.
Propagation of chlorination of methane
free radicals are used and created in a chain
Cl. attacks the methane molecule — Cl. +CH4 —> .CH3 + HCl
the new methyl free radical .CH3 can attack another Cl2 molecule —- .CH3 + Cl2 —> CH3Cl + Cl.
the new Cl. can attack another CH4 molecule until all the Cl2 or CH4 are all wiped out
Termination of chlorination of methane
Termination - free radicals are mopped up.
if two free radicals join togather they form a stable molecule
heaps of possible termination reactions can occur
Cl. + .CH3 —> CH3Cl
.CH3 + .CH3 —> C2H6
.Cl + .Cl —> 2Cl
limitation of radical substituation
Further substitution could occur if another chloride radical collides with the product of propagation.
If the carbon chain is longer, a mixture of products forms as substitution occurs at different positions along the chain
alkenes are unsaturated because
they can make more bond with extra atoms in addition reaction.
what is a sigma bond
it is formed when 2 s orbitals overlap
these overlap in a straight line - giving the highest possible electron density between 2 nuclei - single covalent bond
high electron density means strong electrostatic attraction between the nuclei and shared pair of electrons.
means they have high bond enthalpy - strongest type of covalent bond
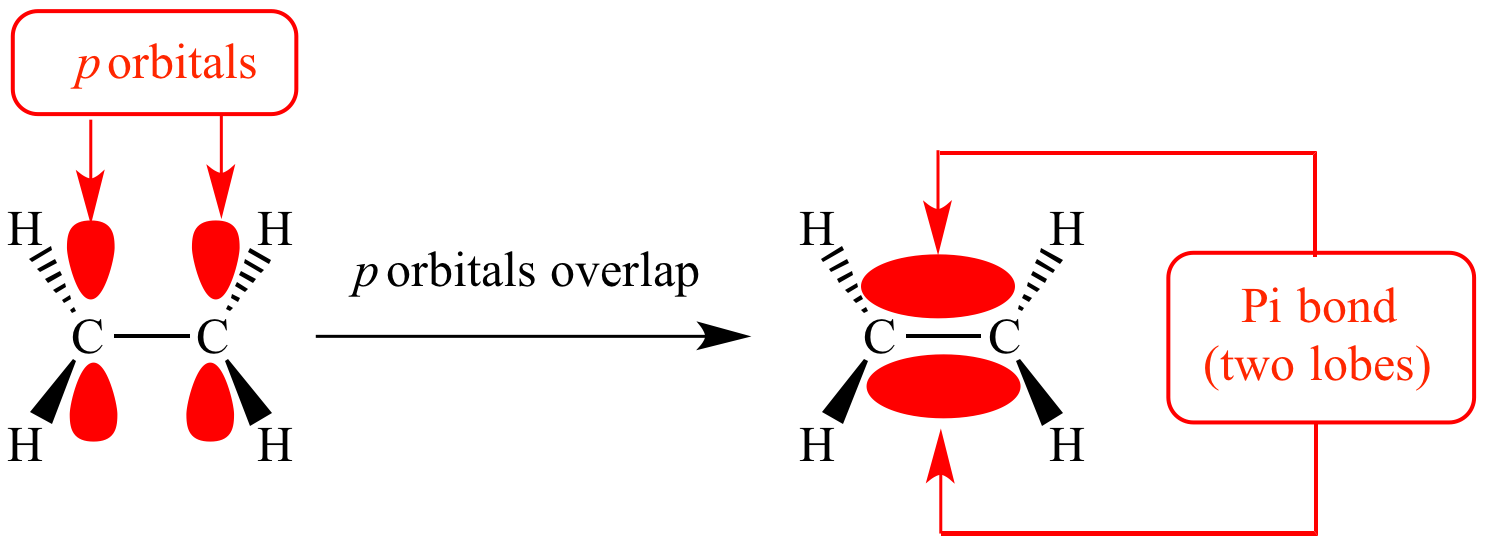
what is double bonds made up of
sigma bonds and pi bonds which makes alkenes more reactive due to the double bond
the C=C bond has 4 electrons so high electron density with pi bonds above and below meaning they are likely to get attacked by electrophile.
because C=C is very reactive handy starting point to making organic compounds e.g. petrochemical.
what is pi bond
sideways overlap of two adjacent p orbitals
got 2 parts to it one above and below the molecular axis
these p orbitals are dumb bell shaped
weaker bonds than sigma bonds as the electron density is spread out above and below the nuclei
means the electrostatic attraction between the nuclei and shared pair of electrons are weaker
so they have relatively low bond enthalpy.
shape of an alkene
they are trigonal planar shape 120 degrees
the 3 regions of electron density around each carbon atom means that they will repel each other as far apart as possible
the C=C and the atoms bonded to these carbons all lie in the same planar
in the imagine that is planar but if one of the H is replaced with CH3 then it is non planar
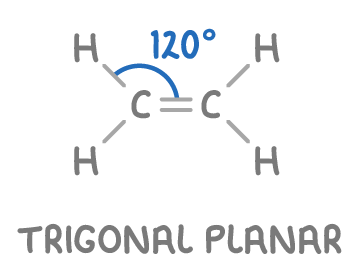
why cant C=C atoms rotate
this is because the pi bonds locks the carbon atoms in position and prevents them from rotating around in the double bond
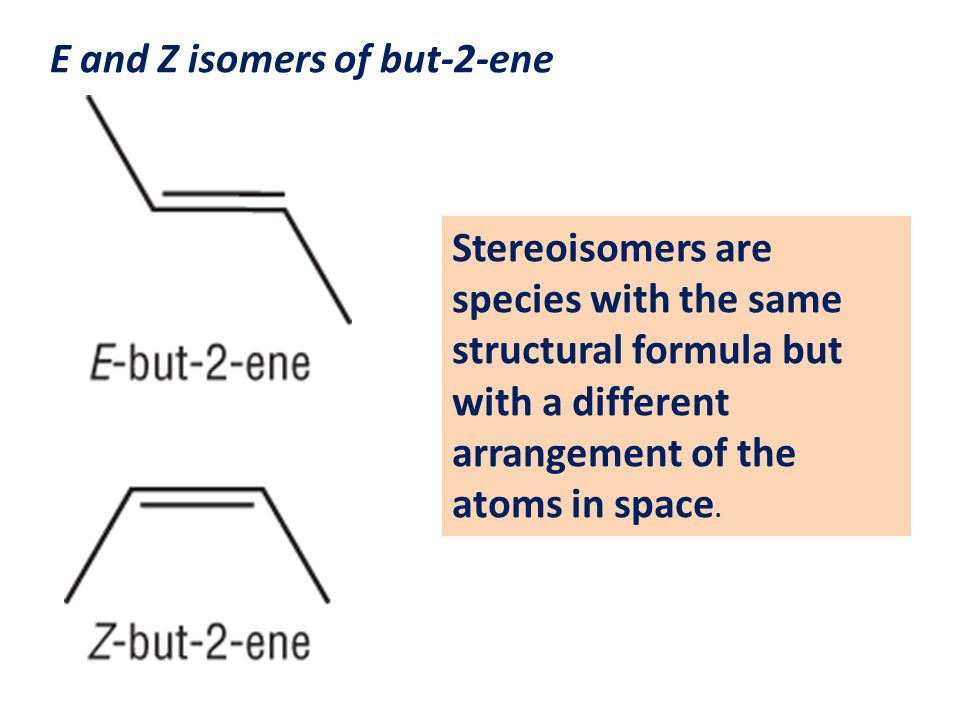
define stereoisomers
compounds with the same structural formula but with a different arrangement in space
define E/Z isomerism
an example of stereoisomerism, in terms of restricted rotation about a double bond and the requirement for two different groups to be attached to each carbon atom of the C=C group
E = enemies Z = same side
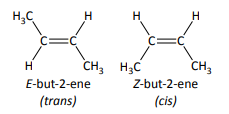
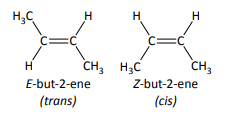
define cis/trans isomerism
a special case of E/Z isomerism in which two of the substituent groups attached to each carbon atom of the C=C group are the same
E = trans
Z = cis
only consistently correct when there is an H on each carbon atom of the C=C bond
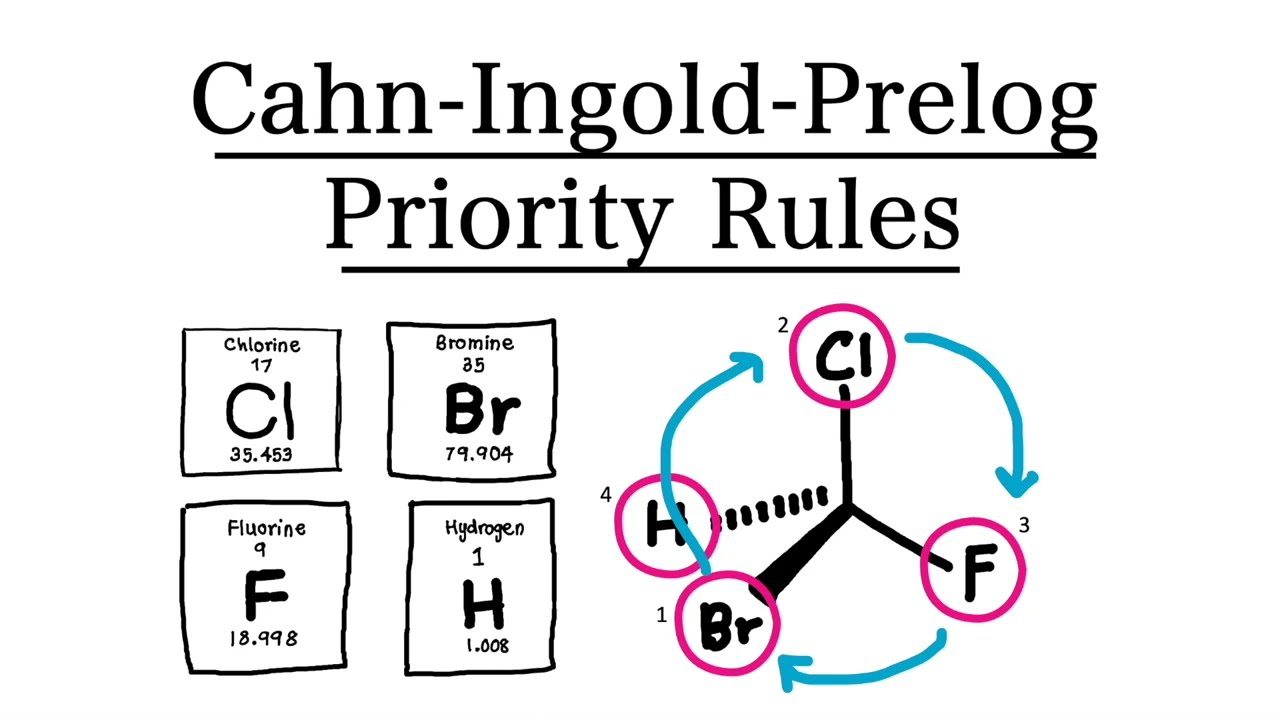
using Cahn-Ingold Prelog priority rule to identify E and Z stereoisomers
in this system atoms attached to each carbon atom in a double bond are given priorities based on their atomic numbers
in the groups of higher priority are on the same side of the double bond = Z isomer
if the groups are placed diagonal across the double bond = E isomer
skeletal formula for but-2-ene
other way around for skeletal
Z = same side
E = enemies