BIO100 Nutrition Reading #1
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Neutrons and Protons
Subatomic particles with around equal mass are located in the nucleus of the atom.
The number of protons determines the chemical element.
Electrons
A subatomic particle with very little mass that orbits the nucleus of an atom at high speeds. These are orbits are very specific called electron shells.
Isotopes
Variations of elements that vary in the number of neutrons and are named by their atomic mass. Protons only determine the identity of the element.
How many neutrons and protons are in nitrogen-15?
15 refers to the atomic mass. Since nitrogen has 7 protons, there must be 8 neutrons to make an equal mass of 15.
Ions
An atom or molecules that has gained or lost one or more electron, creating an electrical charge. Atoms are neutrally charged normally.
One extra negative charge = each extra electron
Ionic Bond
The transfer of one or more electrons from one atom to another.
The atom that recieves the electrons becomes negative, the atom that donates it becomes positive.
Chemical Bonds
An attraction between 2 atoms resulting from a sharing of outer-shell electrons or the presence of opposite charges.
- Gives a computer outer electron shell
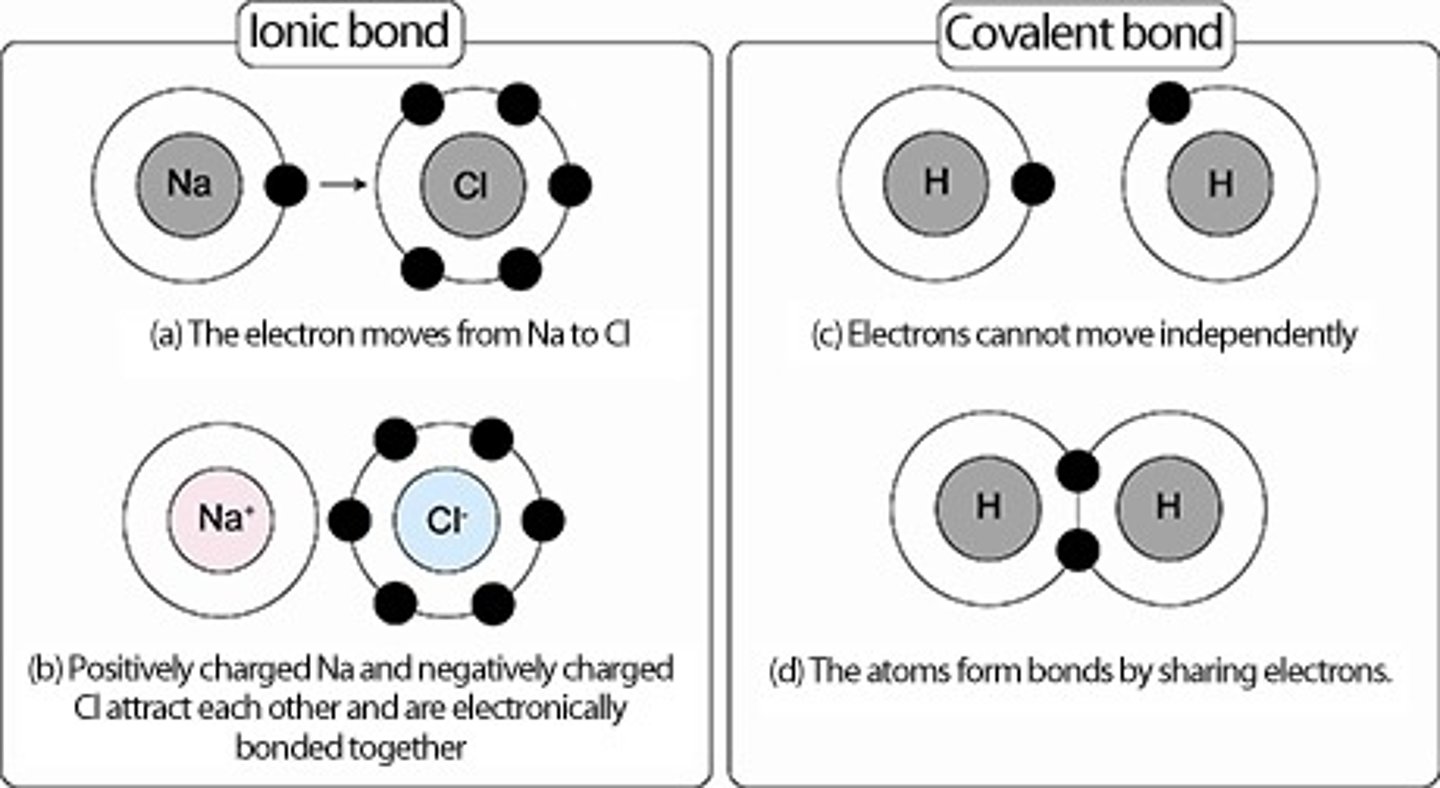
Covalent Bonds
The sharing of one or more electrons. Consists of a pair of shared electrons, one from each electron.
- Holds atoms in molecules.
Single Bond
A single pair of electrons is shared in a covalent bond represented by a single solid line.
Double Bond
If two atoms share two pairs of electrons in a covalent bond represented by two solid lines "=".
Nonpolar Bond
A covalent bond where the sharing of electrons is equal between the two atoms.
Polar Bond
A covalent bond where the sharing of electrons is unequal because one electron may be more strongly attracted to one atom than the other. This creates one end to be positively and the other to be slightly negative.
Hydrogen Bond
A weak chemical bond formed when a partially positive hydrogen atom from one polar molecule is attracted to the partially negative atom in another molecule.

Core Question:
What kind of bond holds the atoms together within a molecule of water? What kind of bond acts between molecules of water to hold them together?
Polar covalent bonds; hydrogen bonds.
Organic Compounds
Molecules that contained carbon bonded to other elements.
Carbon is special because it can bond with up to four other atoms.
Functional Groups
Sets of atoms that are attached to the carbon skeleton. Found in almost every organic compound. Often determine the overall properties of it.
What are the four classes of large molecules important to life?
Carbohydrate, lipids, proteins, and nucleic acids.
Examples of Carbohydrates
Cellulose: large, complex. Forms much plant structure.
Glucose: a sugar that acts as an energy source for all living cells.
Examples of Lipids
Coconut Oil: Rich in fat and serves as an important dietary staple.
Cholesterol: Circulates in the bloodstream and acts as an ingriedent for steroid hormones.
Examples of Proteins
Hexokinase: An enzyme, protein that helps drives chemical reactions in most living cells.
Examples of Nucleic Acids
DNA & RNA
Macromolecules
Large molecules that can have complex structures that make a lot of the body. Straightforward because they are made of repeating smaller building blocks.
- Created and destroyed in similar chemical reactions
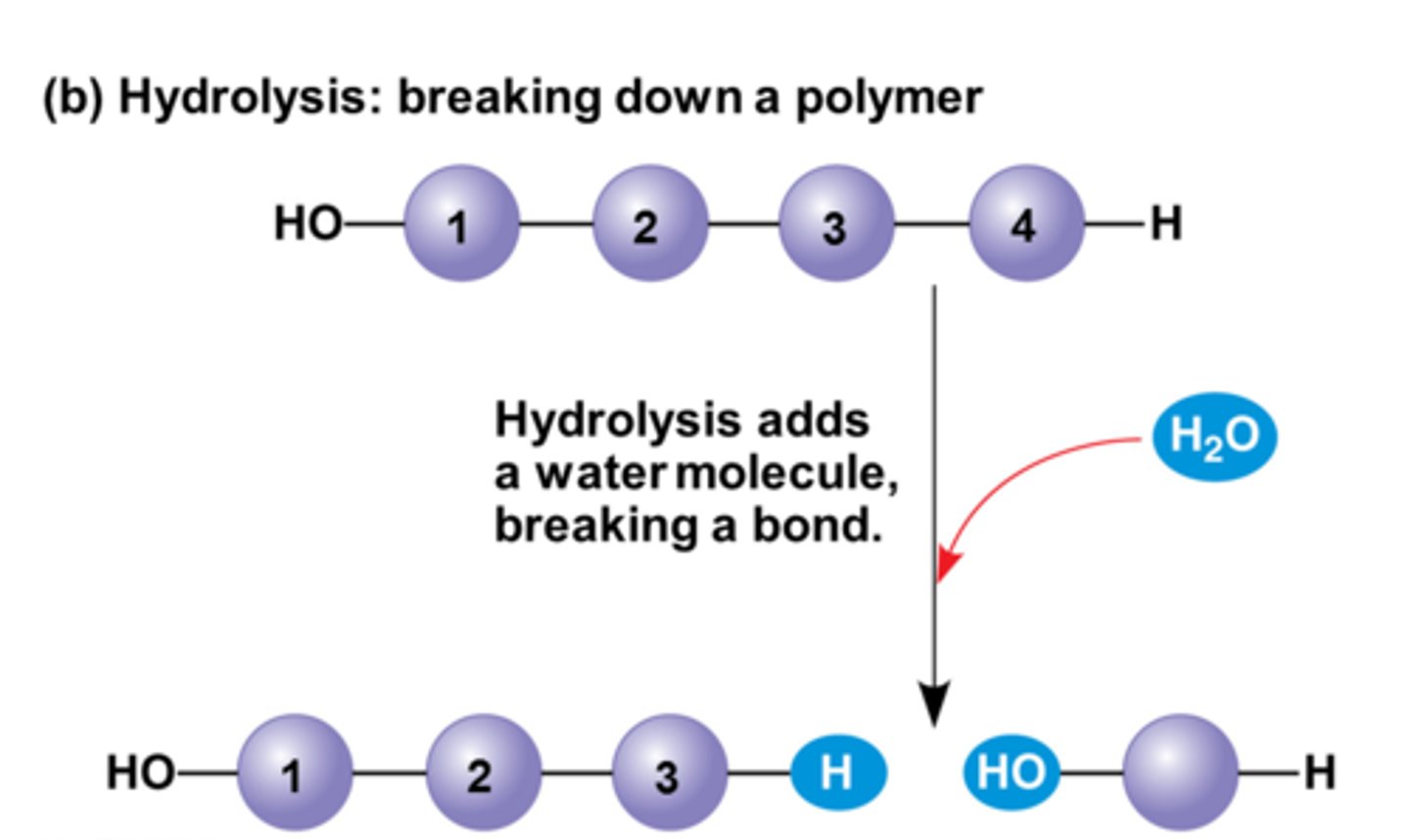
Hydrolysis Reaction
A water molecule is split and its atoms are used to separate a monomer from the rest of the chain.
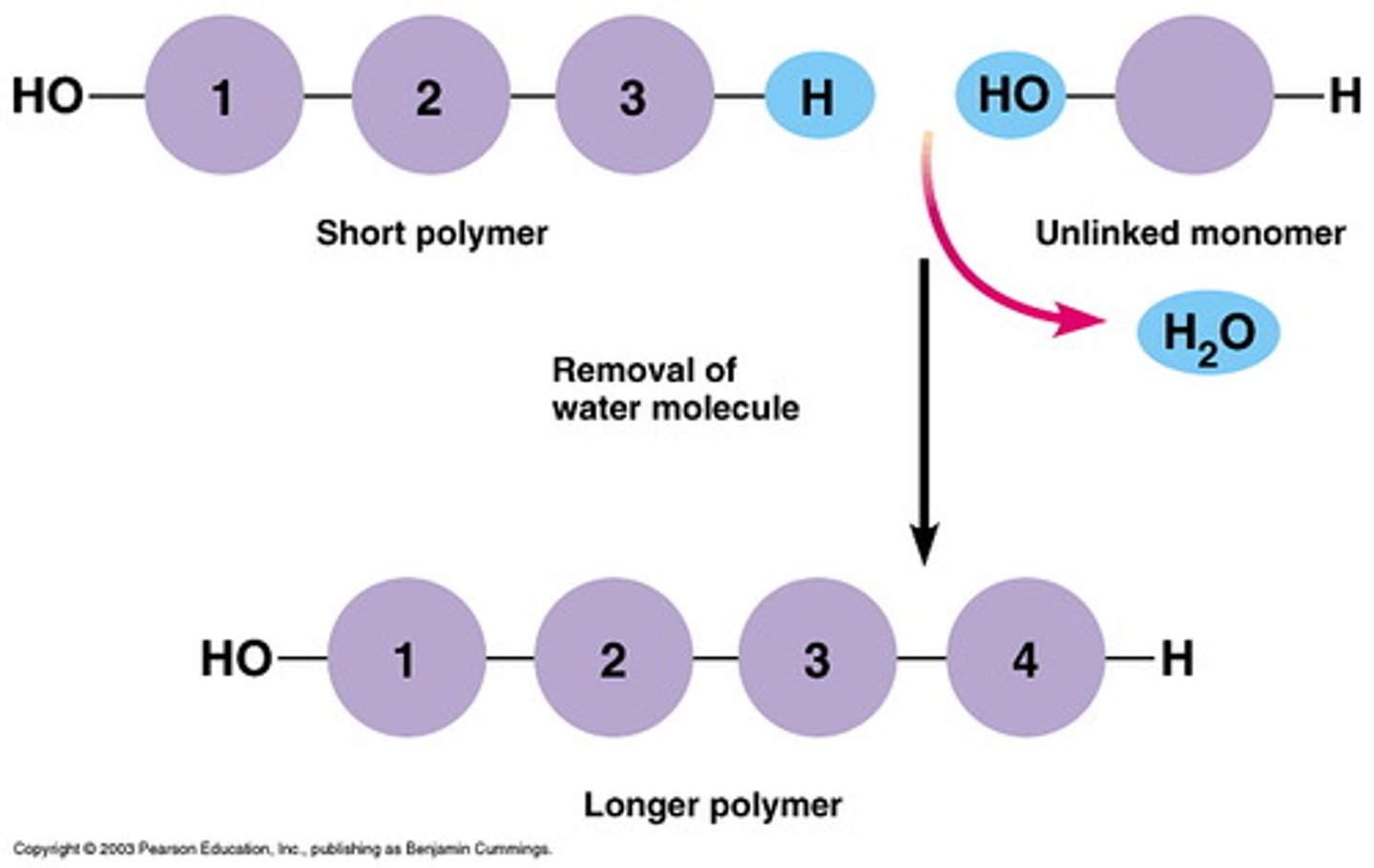
Dehydration Synthesis Reaction
A chemical reaction that links monomers together to form larger ones. The opposite of hydrolysis reaction.
Metabolsim
The sum total of all the chemical reactions that take place in your body.
- Many important metabolic reactions involve breaking down and building up of polymers.
- ex: digestive system breaking down macromolecules you eat into monomers to be used to create muscle proteins.
Core Question:
Why are the building-up reactions called dehydration reactions?
Because a molecule of water is removed from the molecules involved.
Carbohydrates
The starches and sugars present in foods. Molecules constructed from one or more monosaccharide. Important source of dietary energy and structural components.
Monosaccharides
Simple sugars that are the building blocks of carbohydrates.
Isomers
Having the same numbers and kinds of atoms but differ in the arrangement.
- ex: glucose and fructose.
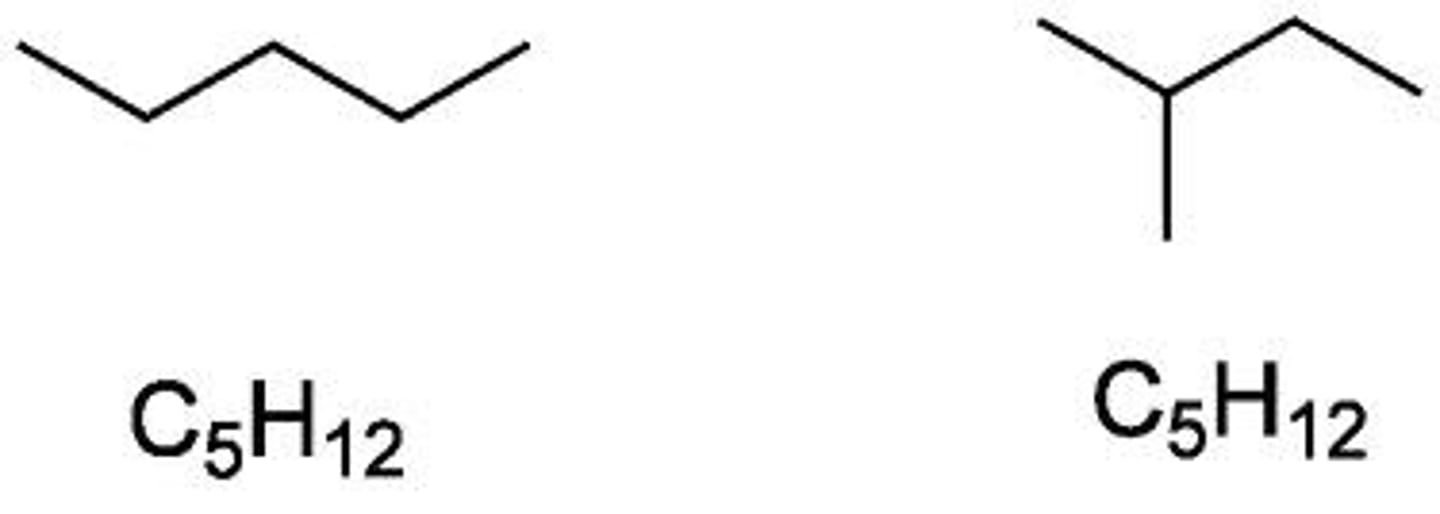
Disaccharide
A double sugar formed by joining two monosaccharides through a dehydration synthesis reaction.
ex: lactose, maltose
Polysaccharides
A complex carbohydrate made by joining many monosaccharides together into a long chain.
ex: made from long chains of glucose monomers
- cellulose, starch, glycogen, chitin
Core Question:
What do starch and glycogen have in common in terms of their structure? How are they different?
They are both large chains made by joining many glucose molecules together, but starch consists of straight chains, whereas glycogen consists of branched chains.
Common Disacharides
1. Lactose (milk sugar) made by combining a molecule of glucose with a molecule of galactose.
2. Maltose (used for brewing and candy) made by combining two molecules of glucose
3. Sucrose (common table sugar) made by combining a molecule of glucose with a molecule of fructose
Chloroplasts
The organelles of photosynthesis found in all plant cells. Require a steady supply of water and carbon dioxide to create sugar (and oxygen/heat as waste).
Mitrochondria
Organelle that provides energy to nearly all eukaryotic cells. Performs cellular respiration using a series of enzymes. Uses oxygen to harvest chemical energy (some ATP) from molecules of glucose (with carbon dioxide, water, and heat as waste)
ATP
Some of the harvested chemical energy made in cellular respiration which is used to power many other processes like growth.
- high energy molecule
- vital to all processes in a body
Core Question:
Do you notice something interesting about the inputs and outputs of photosynthesis and cellular respiration?
The outputs of photosynthesis serve as the inputs for cellular respiration, and vice versa.
Aerobic Respiration
A series of chemical reactions controlled by enzymes in which organic molecules are broken down through the use of oxygen to produce ATP energy.
- requires oxygen
ADP (adenosine diphosphate)
Used to create ATP by being combined with a third phosphate in an energy-consuming reaction. The third one contains considerable potential energy that is released to be used for cell functioning.
ATP (adenosine triphoshate)
A molecule that contains three phosphate groups linked together. Formed by combing ADP with a third phosphate. Acts an energy shuttle that transfers chemical energy from bodily processes that provide energy to ones that use energy.
Core Question:
Trace a molecule of CO2 through your body from its point of origin to where it exits
CO2 is released by mitochondria, exits the cell, enters the bloodstream, goes to the lungs, and is exhaled.
Glycolysis
STEP 1 of cellular respiration:
- takes place in the cytoplasm
- involves the splitting of one molecule of glucose into 2 molecules of pyruvic acid.
- Produces a bit of ATP and some high-energy electrons carried by NADH
Citric Acid Cycle
STEP 2 of cellular respiration:
- takes place in the fluid within mitochondria
- breaks down pyruvic acid to CO2, which is released from the cell.
- produces a bit of ATP and more high-energy electrons carried by NADH and FADH2
Electron Transport Chain
STEP 3 of cellular respiration
- takes place in the mitochondria's inner membrane
- a series of protein molecules
- ATP is synthesized from ADP and phosphate as the high-energy electrons from the previous stages move through the proteins.
- electrons are combined with oxygen to form water.
- produces the most ATP by far.
Core Question:
By the time cellular respiration is complete, what has happened to the carbon atoms in the starting glucose molecule?The h
All of the carbon goes into molecules of carbon dioxide.
Aerobic Cellular Respiration
The harvesting of food energy in the presence of oxygen.
Sugar + Oxygen --> Carbon Dioxide + Water + Energy
Anaerobic Fermnetation
The process of food energy being harvested in the absence of oxygen. Produces a variety of products depending on the organism, but every type recycles molecules of NAD+ that can be used for more glycolysis.
- humans cells cannot run on fermentation alone for long, but some microbial cells can.
Core Question:
What gas is contained within champagne bubbles? Where does it come from?
Champagne bubbles contain CO2, produced through fermentation by yeast.
Core Question:
What molecule serves as the central currency of energy in all your cells? From what molecule is it made?
ATP, ADP