SBI4U - Unit One - Biochemistry
Bonding
Ionic Bonds
dissociate easily in water
one or more electrons transferred between atoms with an electronegativity of greater than 1.7
results in cation and anion
soluble
Covalent Bonds
share electrons between atoms
three types:
polar
unequal sharing of e- pairs
EN between 0.41 and 1.7
eg. H2O
nonpolar
equal sharing of e-
electronegativity of less than 0.4
eg. H2, O2, N2, CO2, CH4
amphiphilic
some larger molecules have parts that are polar and parts that are nonpolar
eg. fatty acids
Note - like dissolves like, so polar dissolves polar, nonpolar dissolves nonpolar
Electronegativity
determines the strength of the bond
F (fluorine) has the highest EN (highest “pull” of e-)
has to do with the distance between valence e- and nucleus
even though e- are being shared, one element may have a stronger “pull”
can lead to the formation of polar molecules
the shape of molecules is related to their polarity
Intermolecular Forces (aka van der Waals Forces)
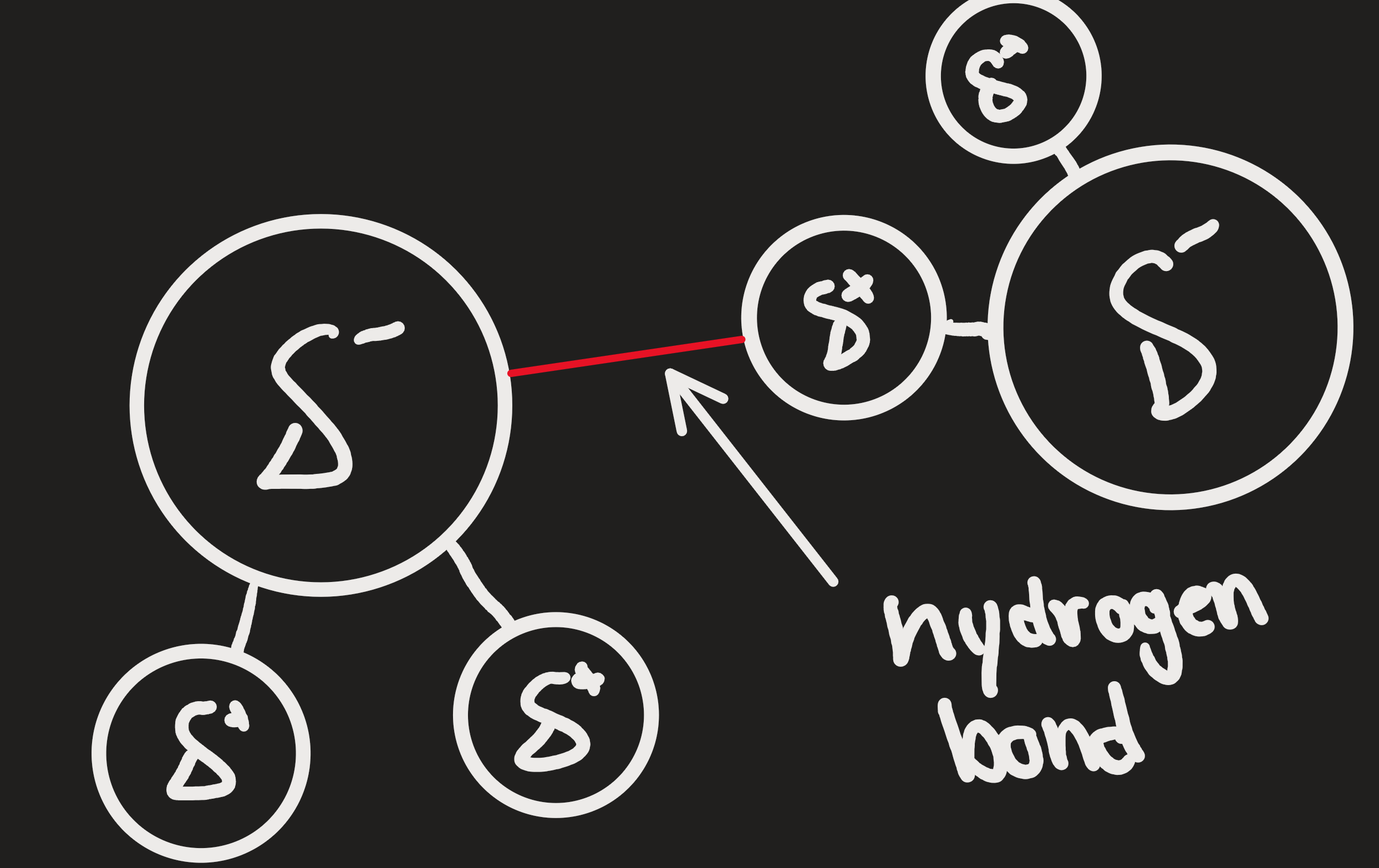
Hydrogen bonds
attractive force between a partially positively charged hydrogen atom and a partially negative charge in another molecule
eg. forces between water molecules
Other van der Waals Forces
weak, momentary attractions of one molecule to the nuclei of another molecule
eg. London dispersion forces, dipole-dipole forces
Chemical Reactions
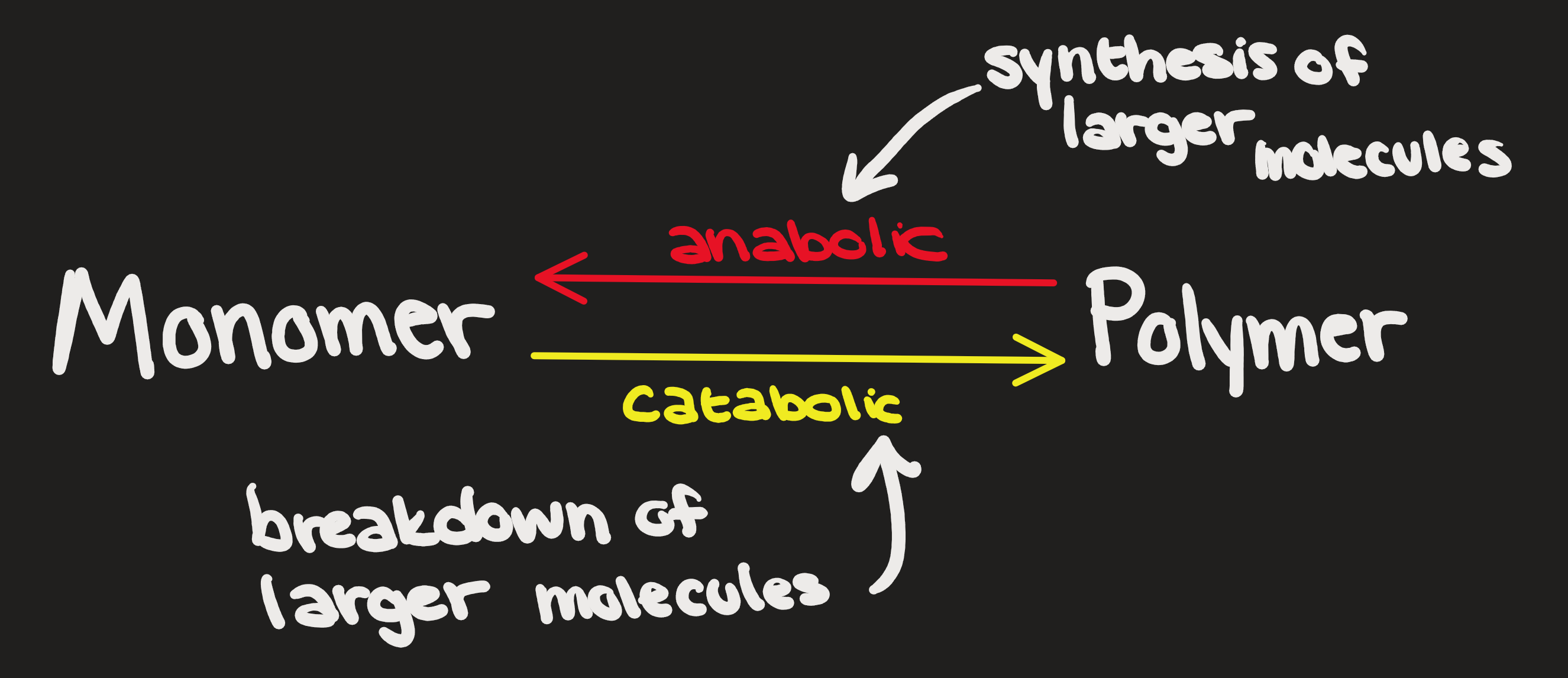
Dehydration Reaction (aka Condensation)
Removal of an OH and H to join smaller molecules and make H2O
eg. occurs naturally in plants, including sugarcane and sugar beets, from which we refine the sugar into pure table sugar
Hydrolysis Reaction
Adding water as OH and H splitting a larger molecule
eg. happens in digestion in your stomach under the influence of the enzyme lactase
Neutralization Reaction
Between acids and bases to form water and salt
eg. digestion - pancreas releases sodium bicarbonate and it is added to the small intestine to increase pH
Redox Reaction
Electrons are lost from one atom and gained by another
eg. Aerobic Cellular Respiration: burning of fuel/glucose to produce energy
LEO - Lose Electrons Oxidation
GER - Gains Electrons Reduction
Water
Polar Covalent Bonds
Oxygen has a higher EN than hydrogen
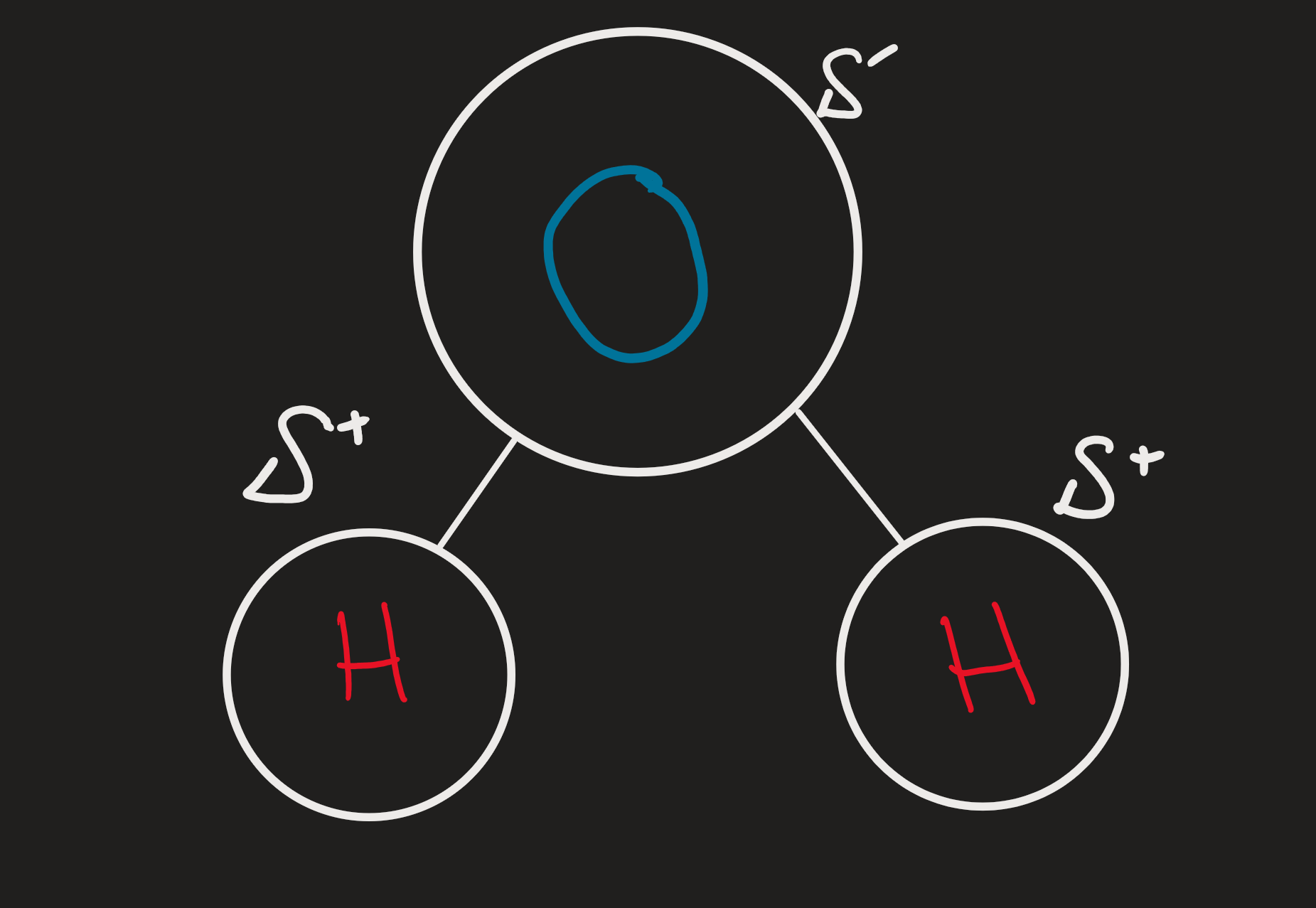
Hydrogen Bonds
Electrons spend more time near the O than the H
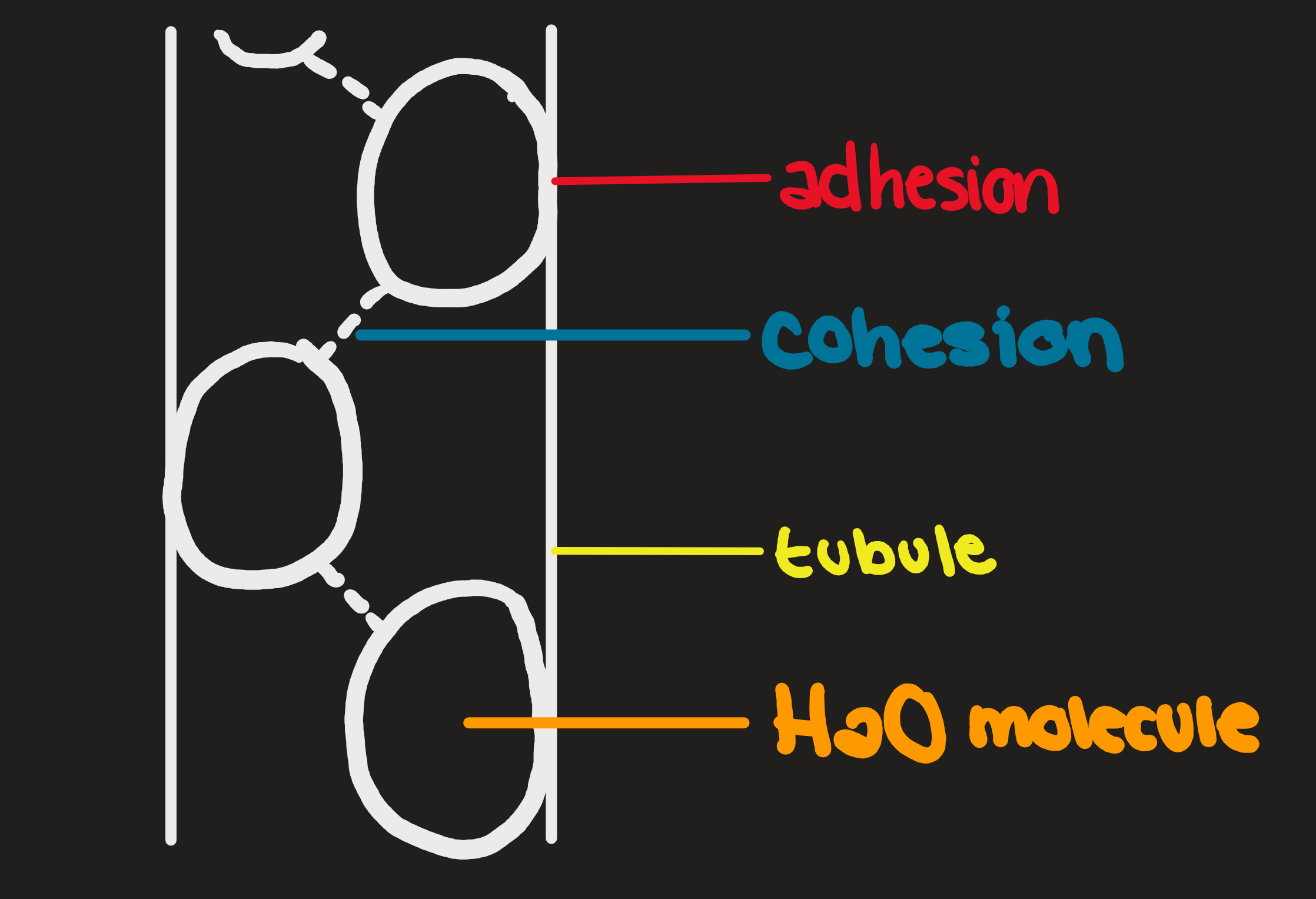
Cohesion
Water molecules are attracted to other water molecules
Adhesion
Water molecules attach to other polar molecules
Capillary Action
Adhesion and Cohesion working together
Climbs inside tubules up to 90m
Surface Tension
Water molecules bond with their neighbours beside and below them
BUT there are more bonds at the surface
Lower Density Solid
Water expands when freezing and becomes less dense
This is why ice floats
Life persists when lakes freeze over because the ice stays on top
Spring/Autumn Turnover
The turnover and shifting of water throughout a body of water
Warm water stays on top, cold water sinks
Oxygenates the deep water and releases sulfurous gases
High Heat of Vaporization
Intenseheat and exercise may generate 1L of sweat per hour and 600 calories burned per litre of sweat evaporated
When sweat evaporates, it pulls the heat with it, cooling us down
High Heat Capacity
Large amounts of heat are required to raise the temperature of water
This is one of many reasons why rising ocean temperatures are so dangerous and concerning
Universal Solvent
Ionic and Polar substances dissolve in water
Properties Summary: (WILL BE ON THE TEST)
Low density solid
Universal solvent
High heat capacity
Heat of vaporization
Adhesion/cohesion
Capillarity
Surface tension
The Carbon Chemistry of Life
Carbon Chains
Carbon atoms are the backbone of biochemistry
Carbon atoms form the basis of the most complex molecules due to their ability to make 4 bonds, allowing for single, double, and triple bonds (as well as combinations of these bonds)
Functional Groups
Commonly found in large molecules
React in predictable ways
Include amino (NH2), carboxyl (COOH), carbonyl (CO), hydroxyl (OH), peptide (CHON), phosphate (PO4)
Macromolecules
Carbohydrates
Lipids
Nucleic Acids
Proteins
Carbohydrates
CHO - made up of carbon, hydrogen, and oxygen
Monosaccharide - Disaccharide - Polysaccharide
Monosaccharides
CHO 1:2:1
Soluble (polar alcohols - OH)
eg. glucose (C6H12O6), fructose
Energy is readily available and easily transported
Disaccharides
Condensation (aka dehydration) reaction occurs when two monosaccharides combine, creating a disaccharide and H2O
Hydrolysis uses H2O to break disaccharides back down into monosaccharides
Glycosidic bond
Transportable energy in plants and animals
eg. sucrose, maltose, lactose
Polysaccharides
Enzymes that digest α linkages can’t hydrolyze β linkages
The cellulose passes through the digestive tract as “insoluble fibre”
Many herbivores, from cows to termites, have symbiotic relationships with microbes that have enzymes to digest cellulose
Polysaccharides - structural
β-linkages have alternating orientation of monosaccharides
examples:
cellulose: long fibrous strings for structural support in plant cell walls
chitin, embedded in proteins, forms arthropod exoskeletons and is used to make strong and flexible surgial thread
Polysaccharides - storage
α gycosidic linkages have uniform orientation of monosaccharides
glycogen - glucose
eg. organelles called leukoplasts store energy in plant roots as starch (amylose, amylopectin)
Summary - Carbohydrates
Functions: energy transport and storage, structural support
Structure: monosaccharides - polysaccharides
eg. glucose, lactose, starch, glycose, cellulose
Functional groups: hydroxyl, carbonyls (aldehydes and ketones) (CARBONYLS ONLY SOMETIMES)
Gycosidic bonds
Lipids
CHO(P)
No monomers and no polymerization
Nonpolar (hydrophobic)
Monoglycerides - Diglycerides - Triglycerides
Glycerol
Fatty acid(s)
Ester bond
Essential unsaturated fatty acids are not synthesized in the human body and must be supplied in the diet
eg. Omega-3 fatty acids
Omega-3 fatty acids have one of their carbon-carbon double bonds at the 3rd carbon atom at the end of their carbon chain
Fat Functions
Store energy
Insulate
Cushioning
Nerve impulse transmission
Phospholipids
Form cell membranes
Amphiphilic
polar head (hydrophilic)
nonpolar tail (hydrophobic)
Other Fats
Messengers (hormones)
blood pressure
sexual characteristics
growth
Protection
waxy layer on leaves and fruit prevents invaders from entering tissue, dehydration
Summary - Lipids
Functions - energy storage, membranes, messengers
Structure - straight chains (fatty acids) and rings
eg. testosterone, cholesterol, beeswax
Functional groups - carboxyl, hydroxyl
Ester bonds
Nucleic Acids
CHONP
DNA - deoxyribonucleic acid
RNA - ribonucleic acid
Nucleodies - monomers
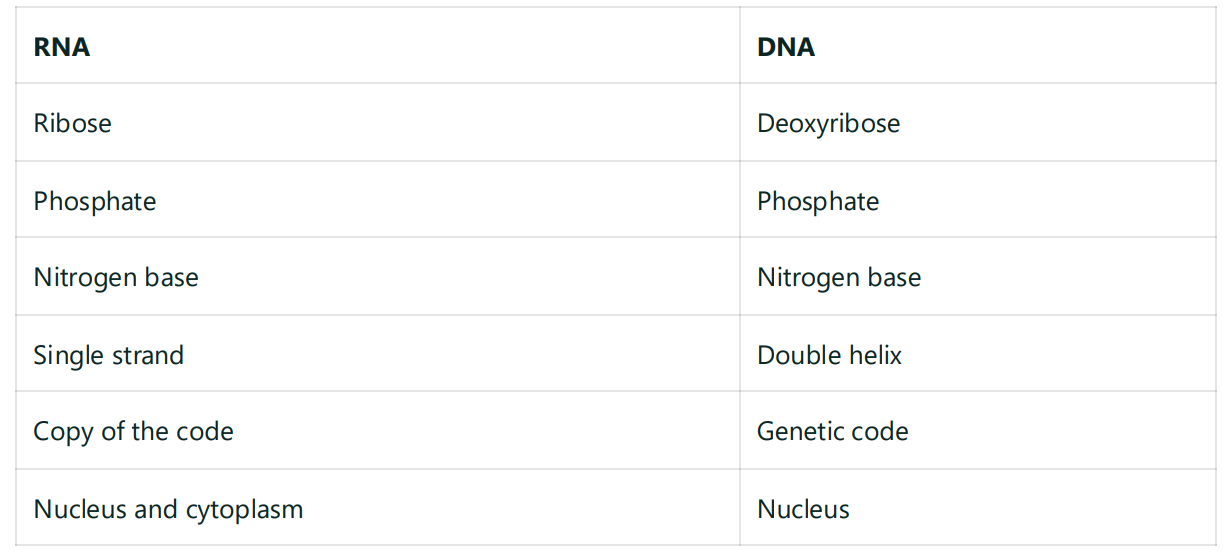
Bonding
Covalent bonds
Phosphodiester bonds
H- bonds
Proteins
CHON(S)
Amino acids (monomers) - dipeptide - polypeptide
Amino Acids
R group
1-20
eg. glycine, alanine
Polar, nonpolar, electrically charged
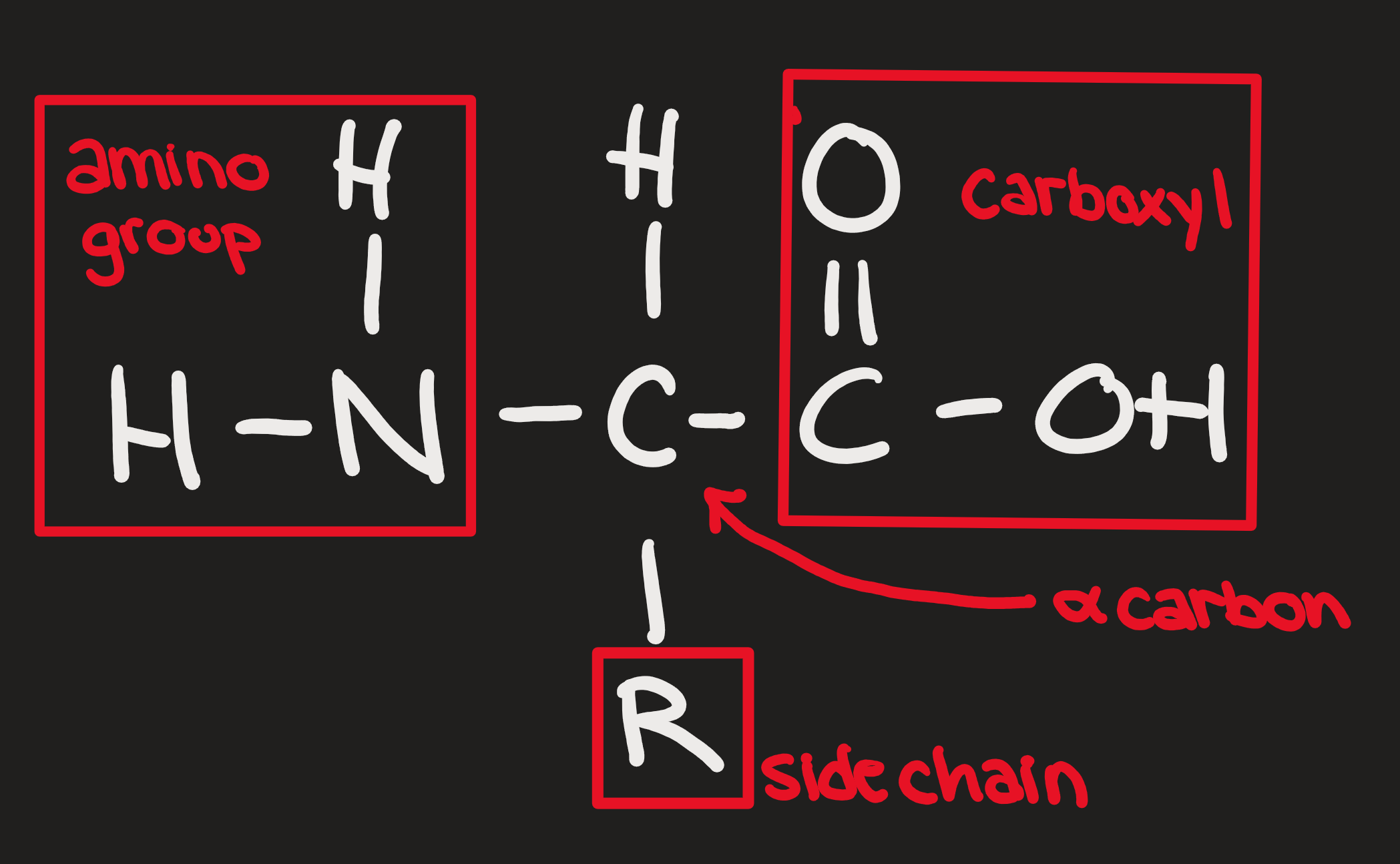
Dipeptides
Polymerization (anabolic)
Condensation (catabolic)
Breaking apart (hydrolysis)
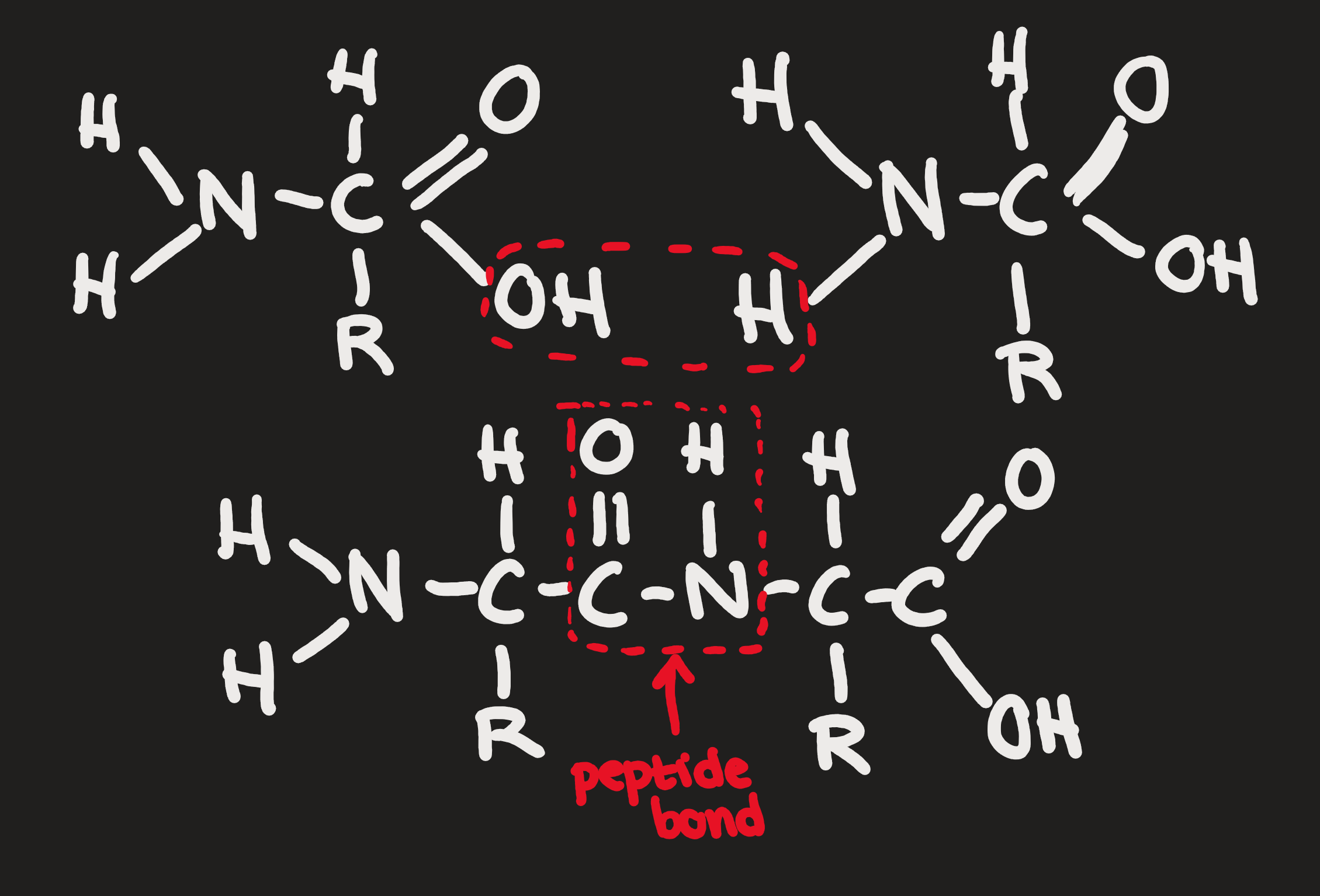
Protein Functions
Enzymes - catalyze reactions
eg. lactase
Antibodies - fight invaders
eg. viruses
Hormones - chemical messengers
eg. insulin
Hemoglobin - transports O2 in RBC
Movement - actin, myosin, etc.
muscles, cilia, flagella
Support - collagen, elastin, keratin
tendons, ligaments, hair, horns, nails, feathers, quills
Nutrient Storage - albumin, amandin
amino acids for developing plant/animal embryos
Protein Organization
Primary
sequence of amino acids coded by DNA (peptide bonds)
Secondary
α helix and β sheet (H- between polar R’s)
Tertiary
3D shape is globular or fibrous (various bonds)
Quaternary
2 or more polypeptides join (becomes a functional protein)
Proteins Denature
Nonfunctional
Bonding in the tertiary structure is disturbed
Caused by:
Temperature
pH
Salt concentration
Summary - Proteins
Functions - transport, movement, messengers
Structure - 20 different amino acids - polypeptides
eg. enzymes, hemoglobin, anitbodies, hormones
Functional groups - amine, carboxyl
Bonds - peptide
EVERYTHING ABOVE THIS POINT IS ON THE UNIT ONE QUIZ
Cell Membranes
Types of Cells
can be prokaryotic
eg. bacteria cells
can be eukaryotic
eg. plant and animal cells
Eukaryotic Cells
organelles are the cell parts
the amount of each type of organelle varies by cell type
eg. muscle cells contain many mitochondria
eg. white blood cells contain many lysosomes
eg. pancreatic cells that make insulin contain a lot of rough ER
The Cell Membrane
Functions
maintain cell shape
allows some things to enter/exit (semi-/selectively permeable)
commication with other cells
show cell identity
Fluid Mosaic Model
flexible
made of many different parts
phospholipid bilayer with protein embedded throughout
molecules are attracted to each other but float freely
Phospholipids
form a bilayer spontaneously
hydrophilic heads associate with water inside and outside of the cell
nonpolar tails form hydrophobic inner layer
Carbohydrates (part 2!!!)
markers that identify the cell
glycoproteins
“person specific” so the immune system can recognize “invaders”
eg. sometimes transplants are rejected because of these markers
glycolipids
“tissue specific” so cells stop multiplying and stay put
eg. metastasized tumors ignore these markers
Cholesterol
contributes to the fluidity of the membrane
reduces membrane fluidity at moderate temperatures, but at low temperatures hinders solidification
Globular Proteins
receptors for communication
transport substances in and out
speed up reactions
anchors cells and their parts
two types
integral
embedded in protein
peripheral
attached to surface
Fibrous Proteins
form a cytoskeleton to maintain cell shape
shape is closely tied to function
Passive Transport
small particles diffuse across a membrane from high concentration to low concentration until an equilibrium is reached
Osmosis
the diffusion of water across a membrane
Simple Diffusion
movement of molecules from an area of [high] to [low] across a membrane
small molecules (O2, CO2)
nonpolar molecules only (steroids, amino acids)
Facilitated Diffusion
requires a specific protein channel
moves down concentration gradient
requires no energy
large, polar molecules (glucose, fatty acids, amino acids)
ions require channel proteins (K+, Cl-, Na+, H+)
Solutions
solvent - substance that dissolves the solute (eg. water)
solute - substance that dissolves in the solvent
Hypotonic
lower concentration of solute than inside the cell
in animal cells, this is dangerous - the cell may burst
in plant cells, the cell membrane pushes against the cell wall
Turgor pressure increases = turgid (firm, healthy)
Hypertonic
higher concentration of solute than inside the cell
in animal cells, the cell shrinks and becomes flaccid
in plant cells, the cell membrane tears away from the cell wall
Plasmolysis - rupture of the membrane occurs, killing the cell
Isotonic
equal concentration of solute inside and outside of the cell
EQUILIBRIUM
Active Transport
the movement of particles from an area of low concentration to an area of high concentration (against the concentration gradient)
uses ATP (cell energy)
eg. a toxic substance outside of the cell will actively be pumped out
eg. micronutrients need to be brought into the cell no matter how low the concentration is
Carrier Proteins
certain membrane proteins use ATP (cell energy) to change their shape, allowing particles to be taken in or out against natural diffusion
Endocytosis
the cell membrane folds around a substance, bringing it into the cell
this folded membrane becomes a vacuole
two types
phagocytosis
cell “eating” - taking in solids
pinocytosis
cell “drinking” - taking in liquids
receptor-mediated endocytosis
substances attach to membrane receptors
this causes the membrane to fold inward creating a coated vessicle
eg. LDL (cholesterol) uptake, glucagon, prolactin, insulin, GH, LH
these are all hormones
Exocytosis
vacuoles containing wastes, to be removed or cell products (eg. proteins) for export
the vacuole approaches the cell membrane, fuses with it, expelling the contents
Enzymes and Energy
Enzyme
biological catalyst that speeds up a chemical reaction without being consumed in the reaction
Active Site
a pocket or groove in an enzyme that binds to a substrate
Substrate
a substance that is recognized by and binds to an enzyme
Anabolic Enzyme
pulls molecules together
Catabolic Enzyme
pulls molecules apart
Induced-Fit Hypothesis
Enzymes
are somewhat flexible, changing shape to better accommodate a substrate
bind to one or more substrates (enzyme - substrate complex)
convert the substrate(s) into one or more products
ready to be reused as soon as products leave the active site
break down between 100 and 40 million molecules per second
Activation energy is lower when enzymes are present
(the energy needed to start the reaction)
Cofactors and Coenzymes
cofactors must bind to an enzyme for it to work
cofactors include magnesium, manganese, iron, copper, zinc, calcium, cobalt
coenzymes (NAD+, NADP+, and FAD derived from vitamins) act as electron carriers
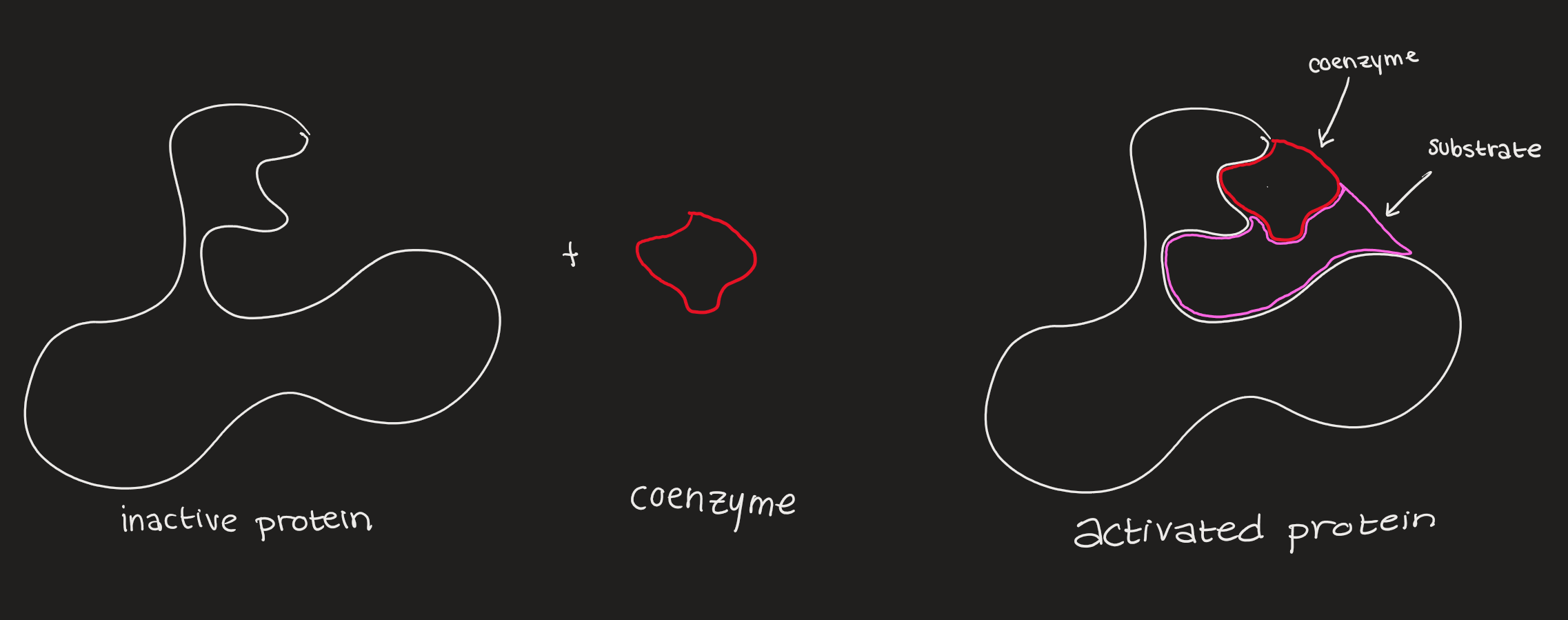
Factors Affecting Enzyme Activity
Temperature
enzymes @ low temperatures - inactive
enzymes @ high temperatures - denatured
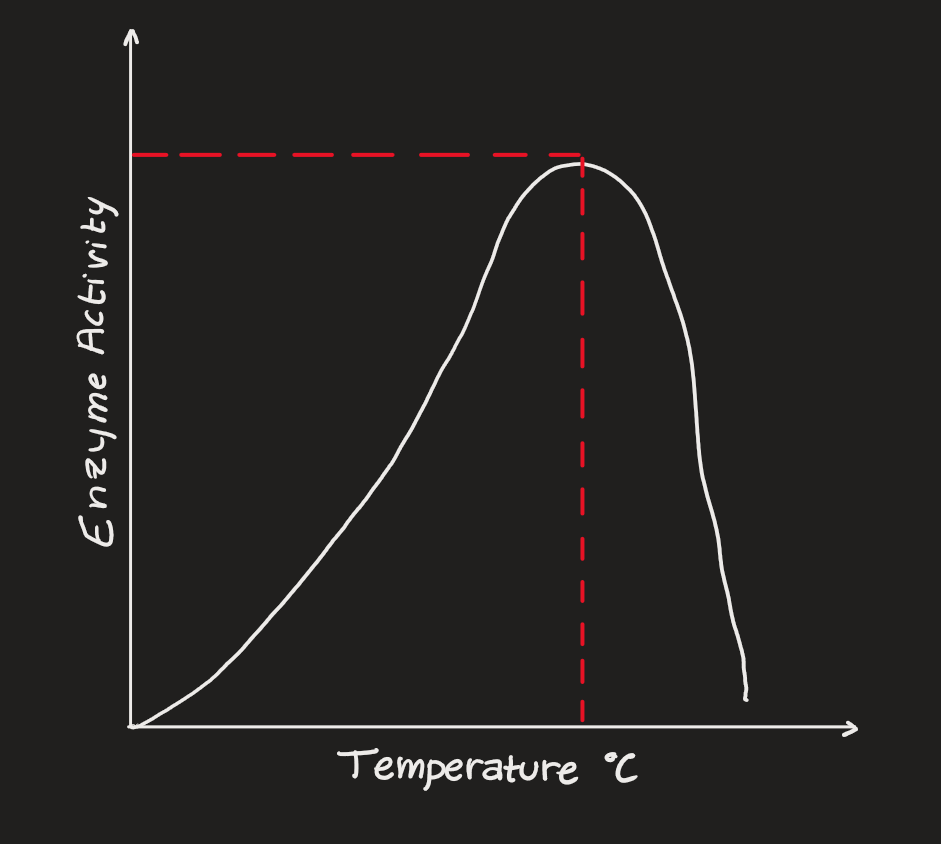
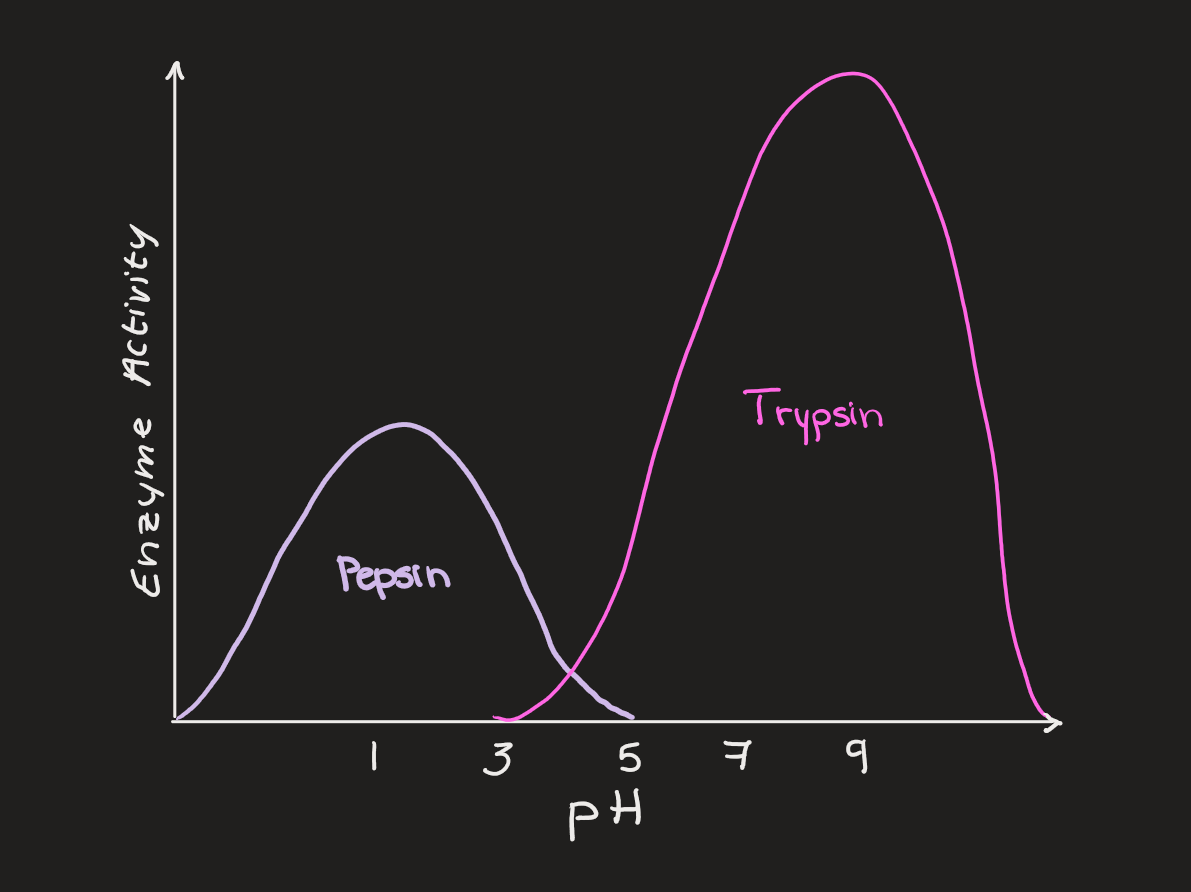
pH
different enzymes have different levels of ideal pH
too basic or too acidic for any given enzyme results in inactivity and denaturation
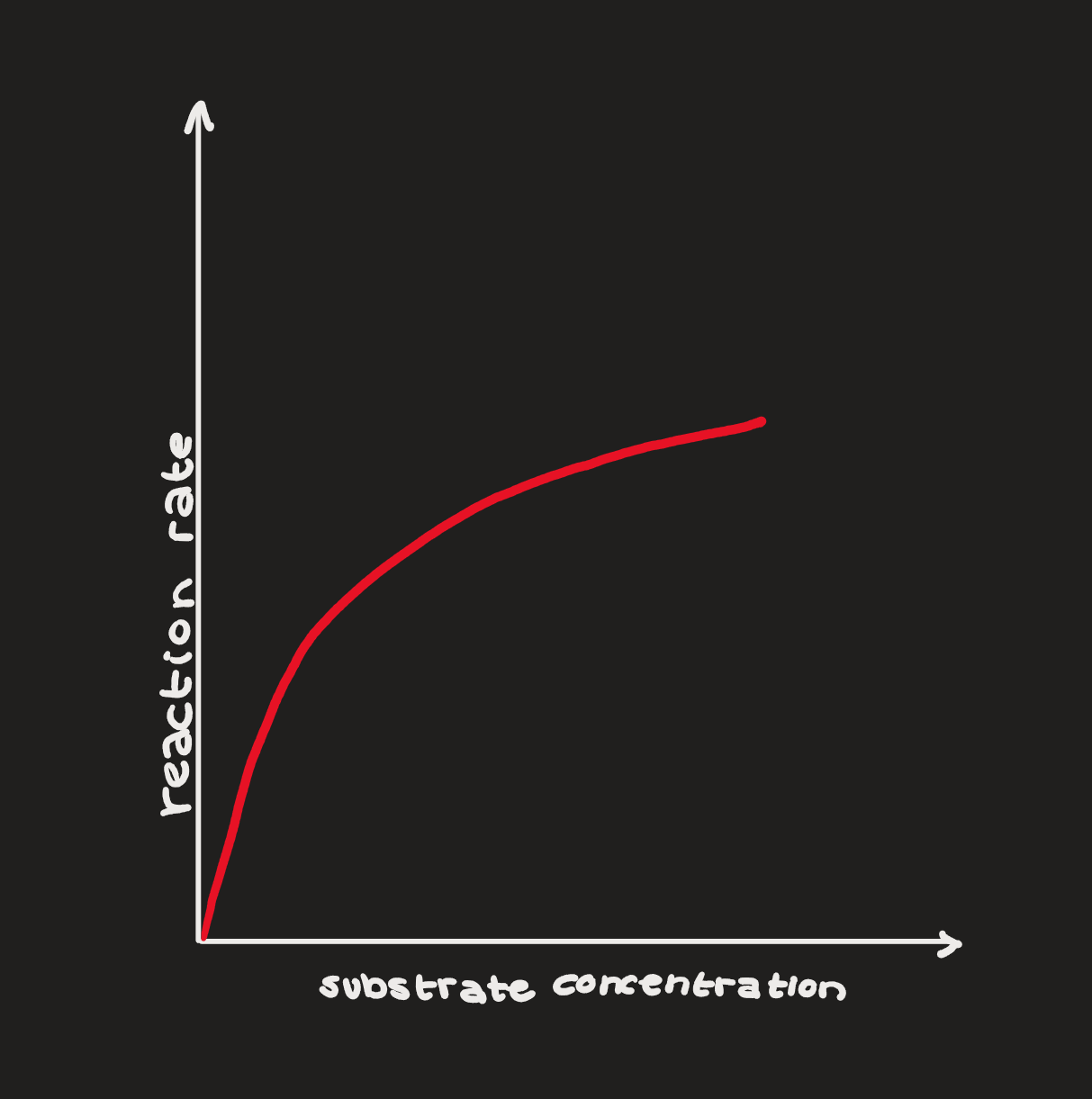
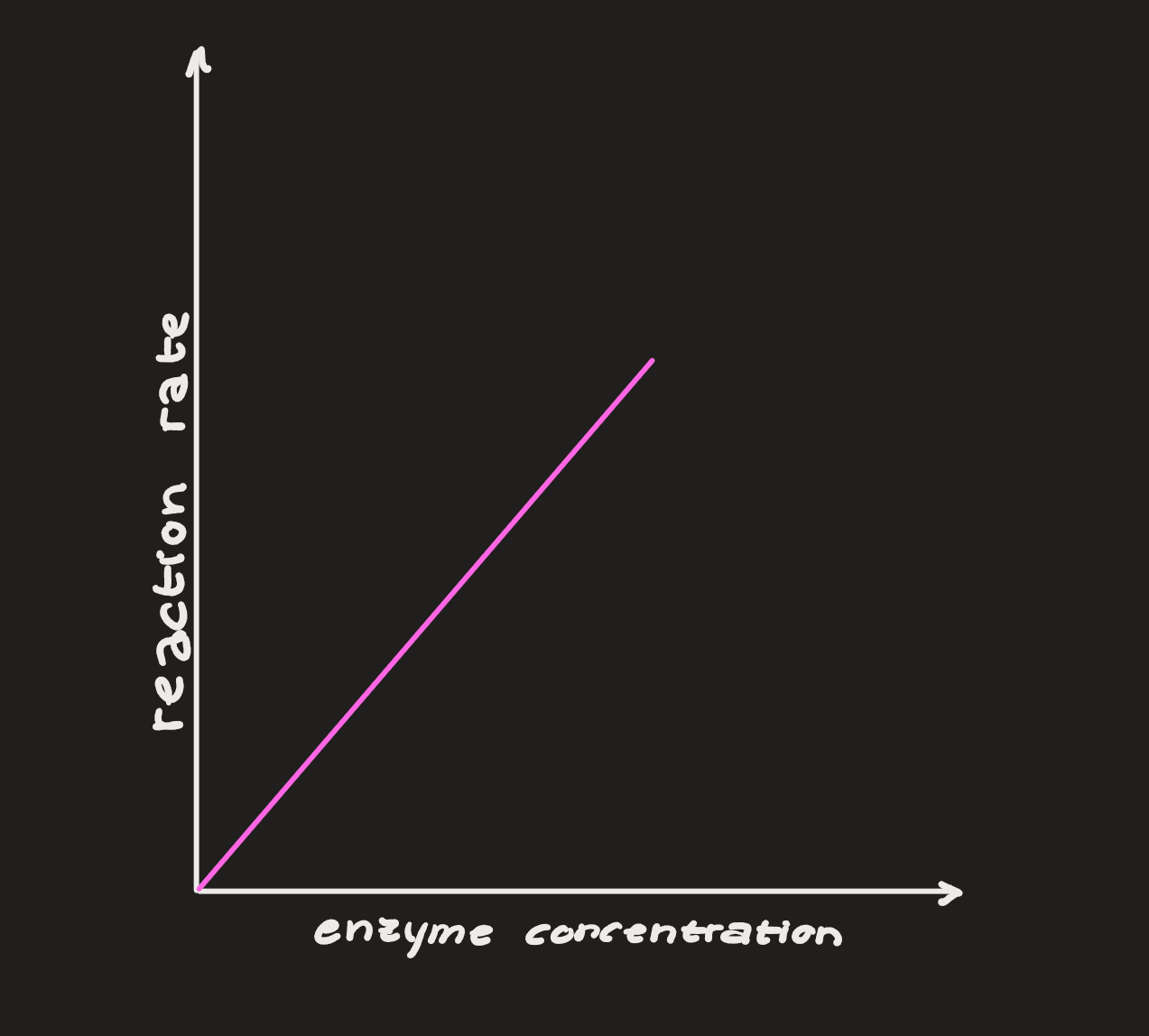
Concentration
enzymes have a certain amount of “work” that they can do (some can break down 100 substrates per second, others can do 40 million)
Allosteric Regulation
Competitive Inhibitors
interference by a molecule (inhibitor) binding to the active site and blocking the substrates
Non-Competitive Inhibitors
a molecule bonds to another place on the enzyme causing a change in the shape of the active site