A&S Ch 4, S&H Ch 101, 103, 187, and RBC in Coagulation MDR
1/310
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
311 Terms
What properties does healthy, intact endothelium have?
Antiplatelet, anticoagulant, fibrinolytic
What is responsible for the antiplatelet properties of the endothelium?
Synthesis of prostacyclin and nitric oxide (NO)
- Both inhibit platelet aggregation
- NO inhibits platelet adhesion
-Vasodilation induced by NO further helps to prevent clot formation by promoting low turbulence blood flow
Platelet aggregation and adhesion are also prevented by enzymes on the endothelial surface that degrade adenosine diphosphate (ADP)
Electronegative charges on endothelium and platelets physically prevent adhesion
Endogenous heparin-like substances are present on the endothelial surface, contributing substantially to anticoagulation
Glycosaminoglycans act as cofactors for antithrombin (AT) which inactivates thrombin and coagulation factors VIIa, IXa, Xa, and XIa
Endothelial cells express thrombomodulin, tissue plasminogen activator (tPA), and tissue factor (TF) pathway inhibitor (TFPI), contributing further to anticoagulation
What is the immediate response of the blood vessel to injury?
Vasoconstriction
- Mediated through local signaling from damaged endothelial cells, perhaps through interruption of the release of endothelial-derived relaxation factors
When vessel injury occurs, endothelial cells can express tissue factor and downregulate expression of thrombomodulin, becoming procoagulant
Activated endothelial cells release von Willebrand factor from Weibel-Palade bodies, promoting platelet adhesion
Primary Hemostasis
Interaction of activated platelets with the exposed subendothelium of blood vessels is the basis of primary hemostasis
Platelet adhesion is mediated by expression of P-selectin on the activated endothelium and by the platelet receptor GPIba, which attaches to vWF
Once attached to the endothelium platelets rapidly change shape and provide an effective monolayer in the adhesion phase
Results in a primary platelet plug (primary hemostasis) that is responsible for preventing leakage of blood from the minute vessel defects that occur daily If blood flow in this area remains nonturbulent, further platelet aggregation does not occur
With large-vessel disruption, blood flow becomes quite turbulent, resulting in large platelet aggregates coating the exposed endothelium
Activation of platelets results in degranulation of platelet contents, releasing agonists
Thrombin, collagen, ADP, and thromboxane A2 promote platelet activation
After the platelet plug bridges the gap between endothelial cells, prostacyclin, produced by neighboring healthy endothelial cells, prevents unwanted expansion of platelet aggregates by decreasing further ADP release
The activated platelet serves as a congregation site for the coagulation factors via the integrin aIIbB3 receptor
What are platelets derived from?
Cytoplasm of bone marrow megakaryocytes
What do platelets contain?
Dense granules
A-granules
Lysosomes
Dense granules of platelets
Store ionized calcium, ADP, adenosine triphosphate, and serotonin
What is the strongest stimulant for the release of the contents of dense granules?
Thrombin
A-granules of platelets
Largest and most prevalent storage granules
Comprise the majority of the storage capacity of platelets
Contain fibrinogen, factor V, factor VIII, fibronectin, vWF, platelet-derived growth factor, and platelet factor 4
Lysosomes of Platelets
Contain predominantly acid hydrolases
Responsible for degradation of unwanted cellular debris after complete activation of fibrin formation
Secondary Hemostasis
Involves the activation of soluble coagulation factors, ultimately resulting in formation of a stable fibrin clot
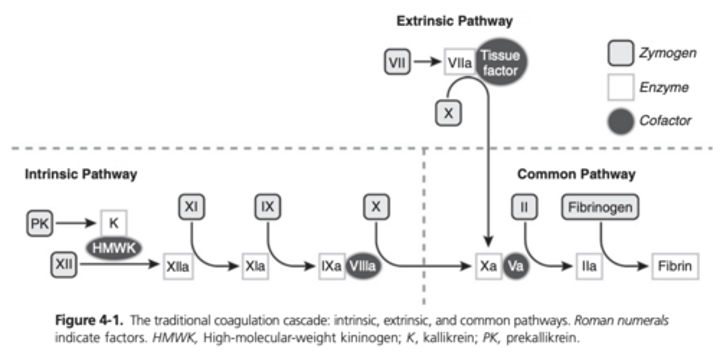
Intrinsic Pathway of the Coagulation Cascade (or Contact Activation Pathway)
Initiated by the activation of factor XII and subsequently factor XI on the surface of activated platelets
Contact proteins, such as high-molecular-weight kininogen (HMWK) and prekallikrein, interact with FXII to accelerate its activation
FXIa activates FIX in the presence of Ca2+
Factor Ixa then binds to procoagulant VIIIa in the presence of Ca2+ It is this complex that activates the common coagulation pathway, marked by the activation of FX
Extrinsic Pathway of the Coagulation Cascade
Primary pathway for initiation of coagulation
Starts with the activation of FVII by TF present in fibroblasts or other TF-bearing cells
TF-FVIIA complex activates FX, leading into the common pathway
Common Pathway of the Coagulation Cascade
Initiated by the activation of FX which in the presence of activated factor V, Ca2+, and a platelet phospholipid, converts prothrombin (FII) to thrombin (Iia)
In the final step of clot formation, FIIa converts fibrinogen to fibrin
FXIIIa stabilizes the fibrin clot by cross-linking strands of fibrin monomer in the presence of Ca2+
Traditional Model of Coagulation Diagram
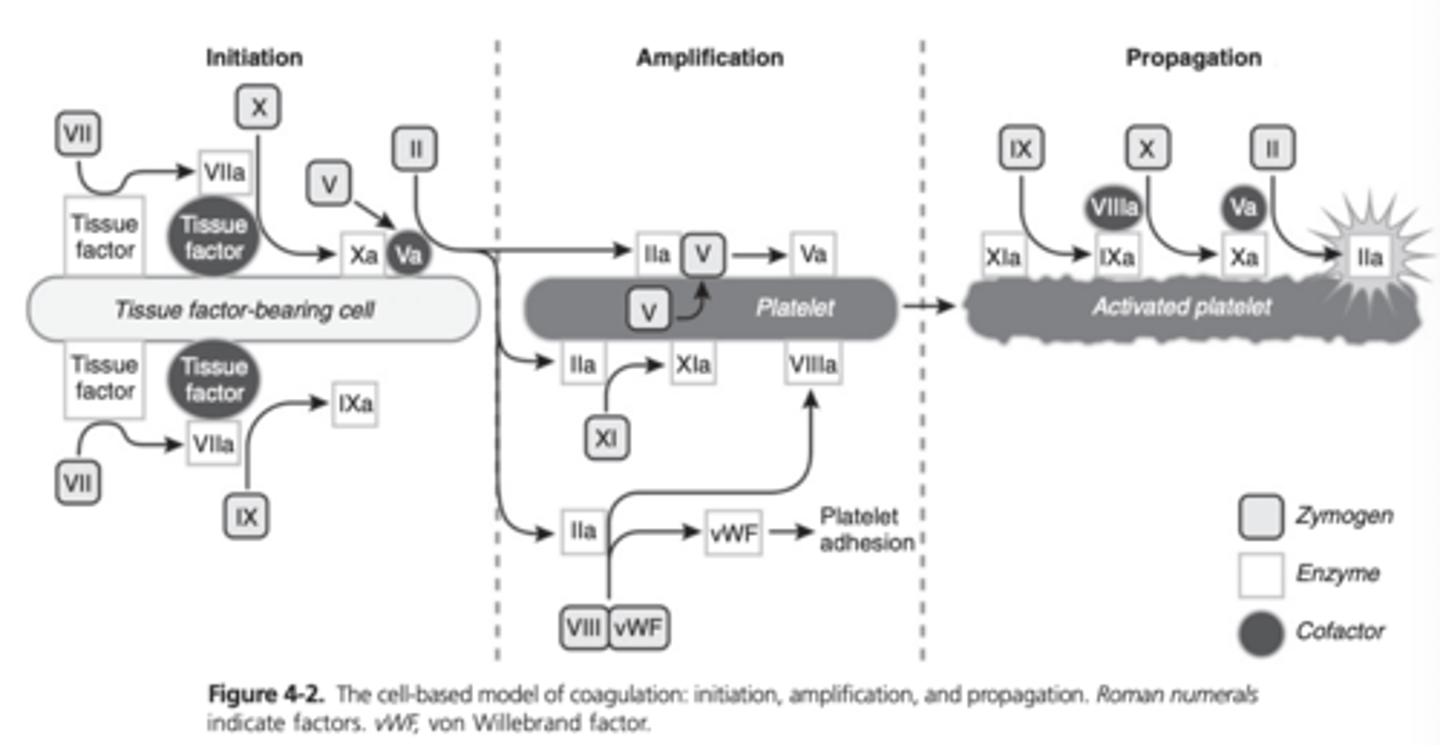
Cell Based Model of Coagulation
Physiologic hemostasis occurs in three overlapping phases: initiation, amplification, and propagation
The intrinsic and extrinsic pathways are still incorporated in this model but the pathways are shown to be highly interconnected
Initiation Phase of Coagulation
When there is disruption of the endothelium, TF-bearing cells such as fibroblasts are exposed to blood, and coagulation is initiated
TF is the primary initiator of coagulation, and the firsts steps of coagulation are limited to the cell membrane
Under inflammatory conditions, TF can be upregulated on the endothelium, monocytes, and other cells and cell particles
FVII circulates in plasma and is available to bind to TF, leading to activated FVII
The TF-FVIIa complex then activates FX and FIX
Although Fxa in plasma is readily inactivated, the membrane-bound Fxa can combine with Fva to produce small amounts of thrombin
Amplification Phase of Coagulation
Once a small amount of thrombin (FIIa) is formed during initiation, the coagulation process can move to the platelet surface
Adherence, activation, and aggregation of platelets along with the accumulation of activated cofactors, constitute the amplification of coagulation
FV present in a-granules
During platelet activation, FV moves to the surface of the platelet
FV is then fully activated by thrombin and FXa
Thrombin cleaves vWF/FVIII allowing vWF to stimulate platelet adhesion
FVIII is bound to the platelet surface and is available to continue the propagation phase of coagulation
FXI is also activated by thrombin on the platelet surface
Propagation Phase of Coagulation
Coagulation complexes assemble on the activated platelet surface and the resulting generation of large amounts of thrombin leads to the propagation of the coagulation process
FIXa is able to reach the platelet surface via diffusion, since it is not inactivated by AT and other plasma protease inhibitors
FIX is also activated on the platelet surface by FXIa
FIXa and FVIIIa combine as the tenase complex and subsequently activate FX on the platelet surface
Fxa and Fva combine to form the prothrombinase complex, which produces a thrombin burst
Cell-Based Model of Coagulation Diagram
Fibrinolysis
Activation of the fibrinolytic system occurs simultaneously with activation of coagulation
Fibrinolysis in conjunction with prostacyclin released by surrounding healthy endothelial cells, inhibits unwanted expansion of the fibrin clot
Where is plasminogen produced?
Kidney and liver
What activates plasminogen? What is it converted to?
tPA and urokinase plasminogen activator (uPA)
converted from plasminogen to plasmin
Action of Plasmin
Degrades fibrinogen and fibrin into soluble fibrin(ogen) degradation products (FDPs) and inactivates other members of the coagulation cascade such as FVa and FVIIIa and actively degrades prekallikrein and HMWK
Degrades fibrin(ogen) and downregulates coagulation
FDPs
Products of fibrinogen or fibrin degradation
Fragment X, Fragment Y, and Fragments D and E
What does plasmin degradation of cross-linked fibrin result in?
D-dimer fibrin degradation product
How are D-dimers removed?
Fragments removed by the mononuclear phagocyticsystem (MPS) of the liver
Accumulation of these fragments indicates increased fibrin production (and degradation) or liver dysfunction
What do increased levels of FDPs, D-dimers, or soluble fibrin monomer in the circulation indicate?
Increased fibrinolysis
Either the result of a thrombogenic process or the patient is in a hypercoagulable state
Principal Inhibitors of Coagulation
Antithrombin (AT)
Heparin
Protein C
Protein S
TFPI
Antithrombin
Responsible for 70-80% of thrombin inhibition in the coagulation system
Serine protease inhibitor
Glycoprotein
Aggressively binds to thrombin Results in a stable thrombin-AT (TAT) complex Removed by the reticuloendothelial system
Cofactor heparin causes a conformational change at the arginine site of AT which dramatically increases its ability to interact with thrombin
Also capable of neutralizing FXIIa, FXIa, Fxa, and FIXa
AT-heparin complex slowly inactivates FVIIa
Where is antithrombin produced?
Liver and endothelial cells
Heparin
Highly sulfated glycosaminoglycan
Ranges in weight from 3-30 kDA
Causes a conformational change in AT, which increases the activity of AT 1000-fold
Decreases fibrin-generated fibrin formation significantly
Releases TFPI from endothelial cells, thereby liberating one of the most effective inhibitors of the FVIIa-TF complex
Where is heparin produced?
Mast cells located in the lung, liver, kidney, heart, and gastrointestinal tract
Protein C
Vitamin K-dependent zymogen
Primary inhibitory action of Fva and FVIIIa
Activated by thrombomodulin-thrombin complexes Reaction potentiated by the endothelial protein C receptor which is located mainly in large vessels
When activated protein C is released into circulation, it associates with protein S and is able to inactivate Fva and FVIIIa
Activated protein C is profibrinolytic since it inhibits plasminogen activator inhibitor-1 (PAI-1)
Tissue Factor Pathway Inhibitor (TFPI)
Group of lipoprotein-bound proteins
Heparin enhances release into circulation
In the presence of Ca2+, inhibits FVIIa-TF activation of FX, thereby dramatically decreasing the primary cellular initiator of coagulation
Where is TFPI produced?
Platelets and endothelial cells
Inhibitors of Fibrinolysis
PAI-1
a-2-antiplasmin
a-2-macroglobulin
Thrombin-activatable fibrinolysis inhibitor (TAFI)
PAI-1
Principal regulator of plasminogen through inhibitory effects on tPA and urokinase
Present in endothelial cells
Stored in a-granules of platelets
a-2-antiplasmin
Main physiologic inhibitor of plasmin
a-2-macroglobulin
Alternative inhibitor of plasmin
May inhibit plasmin in a limited fashion, particularly if a-2-antiplasmin is overwhelemed
Thrombin-activatable fibrinolysis inhibitor (TAFI)
Activated by thrombin, the thrombin-thrombomodulin complex, and plasmin
Plasmin can also activate TAFI as a negative-feedback mechanism
Action of Antithrombin
Anticoagulant
Inhibits factors VIIa, IXa, Xa, XIa, XIIa
Does antithrombin increase or decrease inflammation?
Decreases
Action of Protein C
Anticoagulant
Inhibits factors Va, VIIa
Decreases fibrinolysis
Does Protein C increase or decrease inflammation?
Decreases
Action of TFPI
Anticoagulant
Inhibits factor Xa and TF-VIIa complex
Does TFPI increase or decrease inflammation?
Variable
Action of PAI-1
Antifibrinolytic
Inhibits plasminogen
Does PAI-1 increase or decrease inflammation?
Increases
Action of TAFI
Antifibrinolytic
Reduces conversion of plasminogen to plasmin
Does TAFI increase or decrease inflammation
Increases
Clinical Signs Suggestive of Defects in Primary Hemostasis
Mucosal bleeding
Petechiation
Ecchymoses
Epistaxis
Tests of Primary Hemostasis
Platelet count
Platelet function tests
Template bleeding time (TBT)
Platelet aggregation studies
Platelet function analysis (PFA-100)
What is a normal platelet count?
150,000-250,000 platelets/uL
What is considered an abnormal platelet count?
Less than 100,000 platelets/uL
At what platelet count is clinical bleeding seen?
Below 30,000 platelets/uL
When should platelet function tests be performed?
When there are clinical signs of thrombocytopenia with a normal-to-increased platelet count
Template Bleeding Time (TBT)
Performed on the buccal mucosa or on the caudolateral aspect of the forelimb
Will be prolonged with thrombocytopenia, thrombocytopathia, and lack of vWF, and some cases of vasculitis
Has a poor reproducibility and wide reference range in horses
Prothrombin Time
Measures the function of the extrinsic and common coagulation pathways
Platelet-poor plasma is mixed with thromboplastin and Ca2+ and the time to clot formation is measured
Deficiencies in FV, FVII, FX, prothrombin, and fibrinogen can result in prolonged PT
An increase in time by 20% indicates an abnormal test result
Activated Partial Thromboplastin Time
Measures the function of the intrinsic and common coagulation pathways
Performed by adding an activating agent to platelet-poor plasma in a glass tube containing phospholipid emulsion and Ca2+
Deficiencies of FXI, FX, FIX, FVIII, FV, prothrombin, and fibrinogen can result in prolonged APTT
FXII, HMWK, or prekallikrein deficiencies can prolong APTT but are not associated with bleeding tendencies in humans
An increase in time by 20% is usually considered abnormal
Both PT and APTT serve as variables to evaluate secondary hemostasis
Activated Clotting Time (ACT)
Measures the time required for whole blood to clot after contact with diatomaceous earth, simulating the intrinsic and common coagulation pathways
Will be prolonged with deficiencies of FVIII, FIX, prothrombin, and fibrinogen
Anticoagulant Testing
Antithrombin (AT) is the most commonly measured anticoagulant, measured by chromogenic assay in an automated analyzer and results are reported as a percentage of activity
A decrease in AT levels may occur through consumption in states of increased thrombin formation, through protein loss, such a nephropathies or enteropathies, or through failure of adequate production
Decreased AT and protein C levels are associated with hypercoagulability
AT is an acute phase reactant so may be increased with some acute inflammatory conditions
Thrombin-antithrombin (TAT) is an irreversible inactive complex between thrombin and AT TAT levels can be measured using a sandwich enzyme-linked immunosorbent assay Activation of coagulation and the procoagulant state result in elevated plasma levels of TAT
Fibrin(ogen) Degradation Products
Produced by the proteolytic degradation of fibrin(ogen) by plasmin
Routinely cleared by the mononuclear phagocytic system (MPS) and an accumulation of FDPs indicates a failure of the MPS to adequately remove them from the circulation Can be the result of local or systemic hyperfibrinolysis May be indicative of a dramatic increase in clot formation
Usually performed as a semiquantitative test Possible ranges: 0-10 ug/mL 10-20 ug/mL 20-40 ug/mL >40 ug/mL FDPs >10 ug/mL considered abnormal
Fibrinogen
Low levels potentially related to DIC, liver disease, or dilutional coagulopathy Can be measured by the heat precipitation method, von Clauss technique, or automated photometric detection Horses with DIC do not consistently demonstrate a true hypofibrinogenemia but they do have a lower fibrinogen concentration
D-Dimer
Epitope resulting from the plasmin degradation of fibrin Cross-linked dimer of the two smallest fibrin degradation products, fragment D-D
Assay is specific for plasmin degradation of fibrin In contrast to FDPs which indicate degradation of either fibrin or fibrinogen
Can be measured semiquantitatively by latex agglutination or by latex-enahanced turbidimetric immunoassay performed on a standard coagulation analyzer
Increased D-dimer levels indicate increased fibrinolysis or inability to clear the products from the circulation
Can be increased in horses as a physiologic response to the primary disease, a surgical procedure, or a pathologic coagulopathy
Viscoelastic Monitoring
Thromboelastography (TEG), rotational thromboelastometry, and the Sonoclot analyzer are the three currently available analyzers that use viscosity, elasticity, or both to evaluate clot formation in whole or citrated blood samples Evaluate all phases of clot formation and retraction from a single small volume of blood
Inherited Hemostatic Dysfunction
Von Willebrand disease
Thrombasthenia
Hemophilias
Specific coagulation factor deficits
In horses deficits of prekallikrein and FVIII, FIX, and FXI have been reported
Acquired Hemostatic Dysfunction
Diseases associated with hemostatic dysfunction in the horse include severe liver disease, equine infectious anemia, Anaplasma phagocytophilum, equine viral arteritis
Inflammation and Coagulation
Severe inflammation can cause increases in coagulation, decreases in anticoagulation, and inhibition of fibrinolysis resulting in a procoagulant state
Cytokines and endotoxin can induce increased expression of tissue factor on monocytes, macrophages, and microparticles
Endotoxin and proinflammatory cytokines can also activate platelets and induce the release of vWF from endothelium
Levels of antithrombin are decreased as a result of impaired synthesis, increased consumption, decreased production by the liver, and decreased activation by thrombomodulin
Fibrinolysis is impaired because TNF-a and IL-1B can stimulate an increase in PAI-1
Coagulation derangements can actually contribute to further inflammation since AT and protein C have antiinflammatory effects
Activation of protease-activated receptors during coagulation also enhances inflammation through increased production of TNF-a, IL-6, and IL-8
Early (Subclinical) Stages of DIC
There will be clinicopathologic evidence of platelet consumption, coagulation factor consumption, and hyperfibrinolysis
Severe DIC
Can lead to massive fibrin deposition in the lungs, liver, and kidneys, potentially leading to multiorgan failure
Primary diseases that could result in DIC
Neoplasia, sepsis, trauma, severe acute hemorrhage, clostridial myositis, severe endotoxemia associated with acute gastrointestinal disease
Testing Recommended for Diagnosis of DIC
Determination of the platelet count (thrombocytopenia), clotting times (prolonged PT and APTT), fibrinogen concentration (decreased), D-dimer concentration, or FDPs (increased
How many horses with acute colitis had evidence of subclinical DIC?
1/3
How many more times likely to die were horses with subclinical DIC than those without?
8 times more likely
What percentage of horses with large colon volvulus demonstrate subclinical DIC?
70%
Development of prolonged PT, increased TAT, and thrombocytopenia were associated with a poor prognosis
Abnormal Coagulation Tests in Septic Neonates
Prolonged PT and APTT
Increased levels of fibrinogen, FDPs, a-2-antiplasmin, and PAI-1
Decreased levels of AT and Protein C
What percentage of foals with septic shock were reported to have coagulopathy? What percentage of those demonstrated clinical bleeding disorders?
25% of cases
67% demonstrated clinical bleeding disorders including petechiation and epistaxis
Treatment of DIC
Plasma and platelet transfusions are recommended in cases with active bleeding or with a high risk of bleeding
Anticoagulant treatment early in the course of DIC may limit the activation of coagulation Heparin is the anticoagulant most commonly used
Action of Heparin as a Treatment for DIC
Increases the activity of AT, thereby inhibiting thrombin and FXa
Low molecular weight heparin vs unfractionated heparin
Low molecular weight heparin (LMWH) has an average molecular weight of 4.5 kDA compared with an average of 15 KDA for unfractionated heparin (UFH)
LMWH has greater inhibition of Fxa, dose-dependent clearance, and a longer half-life than UFH
UFH inhibits FIIa and FXa, LMWH just inhibits FXa
In horses, administration of UFH has been associated with prolonged APTT and decreased PCV
Regimen for unfractionated heparin
Heparin Ca2+, 150 IU/kg SQ initially, then 125 IU/kg SQ q12h for 3 days, followed by 100 IU/kg SQ q12h
When using sodium heparin, a dose of 40-80 units/kg q12 is recommended
Regimen for low molecular weight heparin
Dalteparin 50-100 units/kg SQ q24h, enoxaparin 40-80 units/kg (0.35 mg/kg) SQ q24h
Indications for Whole Blood Transfusion
Blood transfusion is likely needed during an acute bleeding episode when the PVC drops below 20%, but in acute severe cases transfusion may be needed before there is a significant drop in PCV
Greater than 30% blood volume loss at surgery generally requires transfusion
Under general anesthesia pale mucous membranes with a prolonged CRT, decreasing TS, hypotension, and hypoexemia are better indicators of blood loss
A rise in blood lactate concentration despite volume replacement with crystalloid or colloid fluids may indicate continued tissue hypoxia and a need for blood tranfusion
An oxygen extraction ratio >40% to 50% in the context of blood loss may indicate a need for blood transfusion
For patients with primary thrombocytopenia or thrombocytopathia, platelet concentrates can be given because whole blood doesn't provide enough to treat severe thrombocytopenia
How can platelet concentrates be obtained?
Plateletpheresis or centrifugation using a slow-spin technique
Packed Red Blood Cell Indications
Indicated for normovolemic anemia such as neonatal isoerythrolysis (NI), erythropoietic failure, and chronic blood loss
What is PCV a better transfusion trigger for?
PCV is a better "transfusion trigger" for chronic anemia compared with acute hemorrhage with transfusions suggested for horses with evidence of tissue hypoxia and a PCV less than 10-12%
Plasma Administration Indications
Indicated for the treatment of clotting factor deficiency, hypoalbuminemia, and failure of transfer of passive immunity in neonates
What do fresh plasma and fresh frozen plasma contain?
Fresh plasma and FFP contain immunoglobulins, coagulation factors (fibrinogen, and FII, FVII, FIX, FX, FXI, and FXII) and cofactors (FV and FVIII) and the anticoagulant proteins antithrombin, protein C, and protein S
What is plasma considered if not frozen within 8 hours of collection?
Frozen rather than fresh frozen
Will have reduced FVIII and FV levels
What is the starting dose of fresh frozen plasma for coagulopathy?
4 mL/kg with reevaluation of the coagulation profile to determine response to therapy
How many different equine blood groups are there? How many factors are in these groups?
8 recognized equine blood groups and 30 different factors identified within 7 of these groups
No true universal donor for horses
Ideal Equine Blood Donor
Ideal equine blood donor is a healthy, young gelding weighing at least 500 kg
RBC antigens Aa and Qa are the most immunogenic so the ideal donor should lack the Aa and Qa alloantigens
There are breed specific blood factor frequencies so a donor of the same breed as the recipient may be preferable, especially when blood typing is not available
Horses that have received blood or plasma transfusions and mares that have had foals are not suitable as donors because they have a higher risk of carrying RBC alloantibodies
Donkeys have an RBC antigen known as "donkey factor" which is not present in horses so donkeys or mules should not be used as donors for horses
How soon after transfusion do horses develop alloantibodies?
Horses can develop alloantibodies within 1 week of transfusion so blood typing and crossmatching are recommended before a second transfusion is performed A second blood transfusion may be performed safely within 2-3 days of the first transfusion without a blood crossmatch
Blood Typing and Crossmatching
Blood typing and alloantibody screening can be used to help find the most appropriate donor horse for the patient but blood typing is time-consuming and only a few labs offer it so it is rarely a practical method of donor selection
A rapid agglutination method for detection of equine RBC antigens Ca and Aa has been developed that may be a more practical method of pretransfusion testing
Hemagglutination crossmatching is widely available and rapidly performed but will not predict all transfusion reactions Rabbit complement can be added to the reaction mixture to detect hemolytic reactions
Major Crossmatch
Mixing the donor's washed RBCs with the recipient's serum
Minor Crossmatch
Mixing the recipient's red cells with the donor's serum
Can a transfusion still be performed if the minor cross match is incompatible but the major crossmatch is compatible?
Yes
The transfusion can still be performed after washing the donor's RBCs
Half-life of transfused RBCs from blood type and crossmatch-compatible donors
20 days
Half life of fresh autologous blood
50 days