CHEM 1411 – General Chemistry I – Useful Formulas and Constants for the Final
1/19
Earn XP
Description and Tags
Flashcards of formulas and constants for the final exam.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
What is the formula to convert Celsius to Fahrenheit?
°𝐹 = 1.8 × °𝐶 + 32
What is the formula to convert Fahrenheit to Celsius?
°𝐶 = °𝐹 − 32 / 1.8
What is the formula to convert Celsius to Kelvin?
𝐾 = °𝐶 + 273.15
What is the equation relating Energy and frequency?
𝐸 = ℎ𝑣
What is the relationship between frequency, speed of light, and wavelength?
𝑣 = 𝐶 / 𝜆
What is the formula relating Energy, Planck's constant, and wavelength?
𝐸 = (ℎ) 𝐶 / 𝜆
What is the formula for Boyle's Law?
𝑃1𝑉1 = 𝑃2𝑉2
What is the formula for Charles's Law?
𝑉1 / 𝑇1 = 𝑉2 / 𝑇2
What is the formula relating volume and number of moles?
𝑉1 / 𝑛1 = 𝑉2 / 𝑛2
What is the Combined Gas Law formula?
𝑃1𝑉1 / 𝑇1 = 𝑃2𝑉2 / 𝑇2
What is the Ideal Gas Law formula?
𝑃𝑉 = 𝑛𝑅𝑇
What is the formula to calculate density using molar mass?
𝐷 = 𝑀𝑃𝑃 / 𝑅𝑇
What is Avogadro's number?
6.02 × 1023
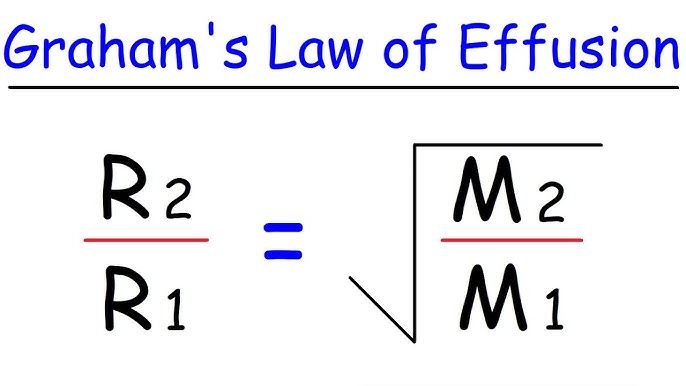
What is the formula for Graham's Law of Effusion?
𝑟1 / 𝑟2 = √(𝑀2 / 𝑀2)
What is the formula for work?
𝑤 = −𝑃∆𝑉
What is the formula for Enthalpy?
𝐻 = 𝐸 + 𝑃𝑉
What is the formula for change in internal energy?
∆𝐸 = 𝑞 + 𝑤
What is the formula for change in enthalpy?
∆𝐻 = ∆𝐸 + 𝑃∆𝑉
How is heat calculated?
𝑄 = 𝑚 × 𝐶 × ∆𝑇
What is the formula for standard enthalpy of reaction?
∆𝐻𝑟𝑥𝑛0 = Σ𝑛∆𝐻𝑓0(𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠) −Σ𝑚∆𝐻𝑓0(𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠)