order reactions
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
What are the units of k for each order reaction?
0 order: k = concentration · time⁻¹
1st order: k = time⁻¹
2nd order: k = concentration⁻¹ · time⁻¹
0 order reaction
Rate = k
Rate ∝ [drug]⁰
Rate is constant.
Rate is independent of concentration
1st order reaction
Rate = k[Drug]
Rate ∝ [drug]
Rate is dependent on concentration
2nd order reaction
Rate = k[Drug]²
Rate ∝ [drug]²
Rate is dependent on the square of the concentration
0 order half life equation:
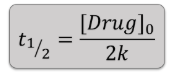
1st Order half life equation:
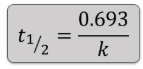
2nd Order half life equation:
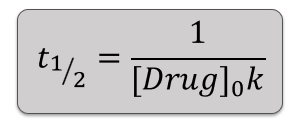
How does increasing initial concentration affect 0 order reactions?
Increasing the initial conc increases the half life
How does increasing initial concentration affect 1st order reactions?
Increasing the initial conc does not change the half life
How does increasing initial concentration affect 2nd order reactions?
Increasing the initial conc decreases the half life
What is removed per unit time in each order reaction?
Zero order: constant amount removed per time
First order: constant fraction removed per time
Second order: rate strongly depends on concentration
Zero order plot
[Drug] against Time
Gradient: -k
![<p>[Drug] against Time</p><p>Gradient: -k</p>](https://knowt-user-attachments.s3.amazonaws.com/b4c38569-86c8-4df3-a512-8c912dccebae.png)
1st order plot
ln[Drug] against Time
Gradient: -k
![<p>ln[Drug] against Time</p><p>Gradient: -k</p>](https://knowt-user-attachments.s3.amazonaws.com/ee1a0a96-127b-42a2-b868-edf5dcf435d6.png)
2nd order plot
1/[Drug] against Time
Gradient: +k
What are the units of k for a 0 order reaction?
Concentration per time
e.g.
mol L⁻¹ s⁻¹
What are the units of k for a 1st order reaction?
Time⁻¹
e.g.
s⁻¹
day⁻¹
What are the units of k for 2nd order reactions?
Concentration⁻¹ · time⁻¹
e.g.
L mol⁻¹ s⁻¹
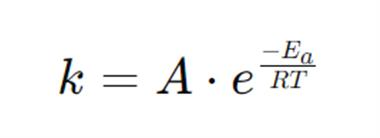
What does everything stand for?
k = Rate constant
A = frequency factor
Ea = Activation energy
R = 8.314 J mol-1 K-1
T = K
What is an Arrhenius plot?
lnk against 1/T
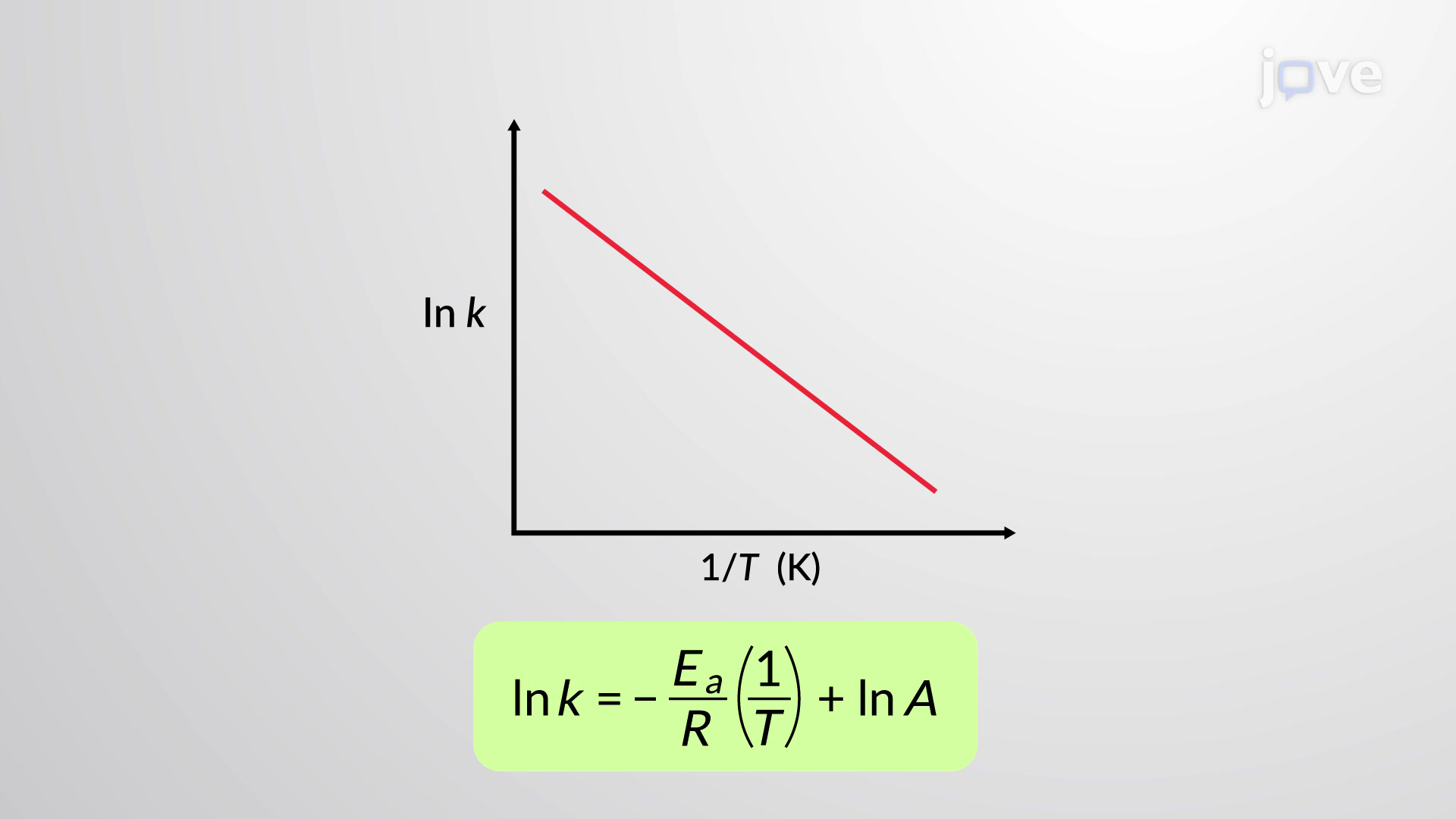
y=mx+c in Arrhenius plot
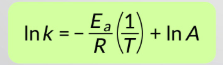
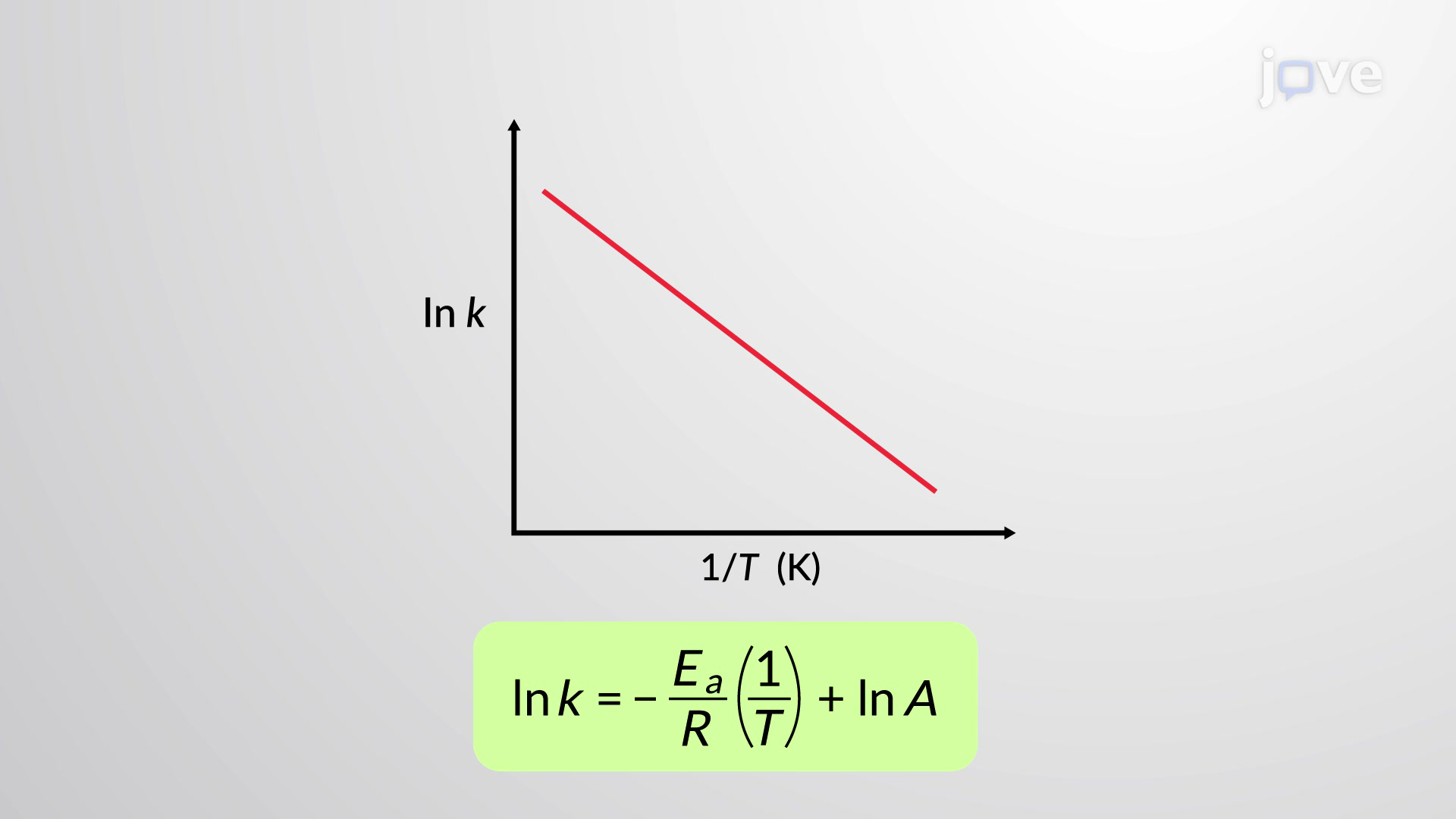
What does the gradient of an Arrhenius plot equal?
−Ea / R
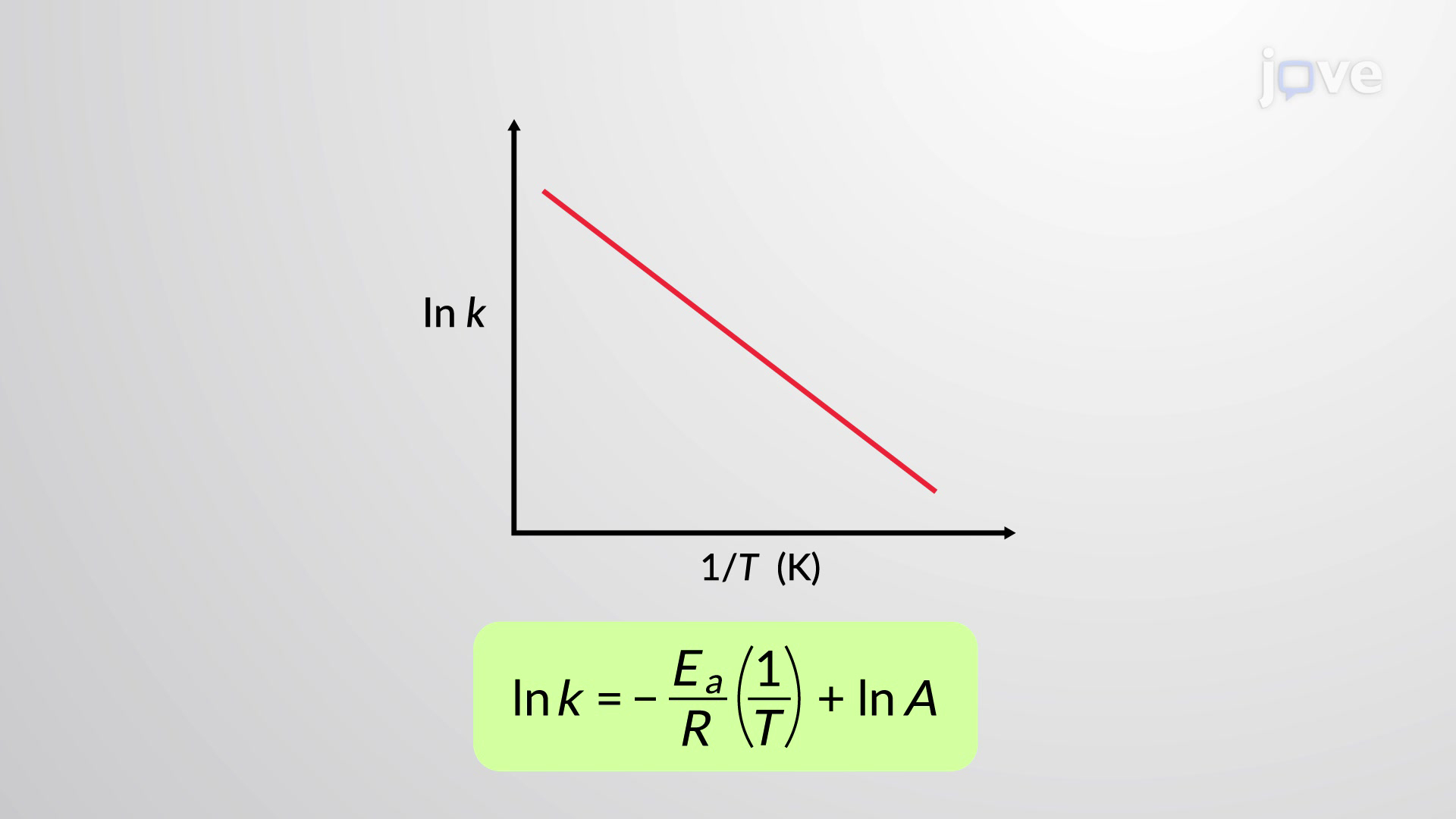
What does the y-intercept (c) of an Arrhenius plot give?
ln A
BCS Class I
High solubility
High permeability
BCS Class II
Low solubility
High permeability
BCS Class III
High solubility
Low permeability
BCS Class IV
Low solubility
Low permeability