Chemistry Exams Common Mistakes
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
River Water is often polluted by phosphates and nitrates. Give the source of these two pollutants.
Fertilisers
Name one environmental effect caused by these pollutants in river water.
Eutrophication.
Describe how insoluble solids are removed from river water.
Filtration.
A compound that leads to deoxygenation of water in rivers.
Sodium Nitrate
Contains an anion with a charge of -1.
Lead Chloride
State the difference between two PHYSICAL properties of a Group 1 or Group 2 Metal And Transition Metals
Group 1 and Group 2 metals are;
Less Dense
Softer
Lower Melting Point
Transition metals;
Higher Density
Harder
Higher Melting Point
Give two reasons why Copper is good for electrical wiring.
Good conductor of electricity, and Ductile (Not Malleable, Ductile.)
What changes does increasing the concentration of a reacting acid do to the rate of reaction and volume of a gas accumulated.
Increasing concentration of an acid increases the rate of reaction, however volume of gas accumulated remains the same.
Ca + Zn2+ —> Zn + Ca2+Explain in terms of the movement of electrons, how calcium acts as a reducing agent in this equation.
Calcium gives electrons to the zinc ions, reducing them while becoming oxidized itself.
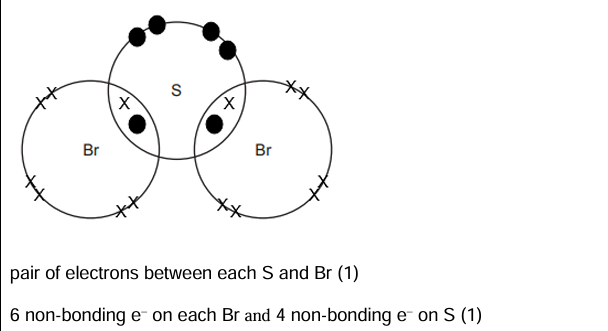
In a Dot and Cross Diagram of Covalent bonds, show the bonding electrons as well as NON BONDING electrons.
A visual representation of shared electrons between atoms, where dots represent electrons from one atom and crosses represent electrons from another, clearly indicating both bonding and non-bonding pairs.
Name the type of monomer to make Proteins.
Amino Acids (Amine N-H2 and Carboxylic Acid -COOH groups)
State the Color of Thymolphthalein in Acids and Bases
Colourless in Acids, Blue in bases.
Define the term Weak in the phrase Weak Acid.
Partially Ionizes in Aqueous Solution.
Define the term Strong in the phrase, Strong Acid.
Completely Ionises in Aqueous Solution.
Describe one method of reducing the amount of sulfur dioxide getting into the air.
Flu gas desulfurization.
Describe one method of making Ethanoic acid by Oxidizing an Alcohol other than Methanol.
Oxidizing an alcohol like an ethanol with an oxidizing agent like Potassium Manganate(VII)
The difference in electrical conductivities of Graphite and Phosphorus.
Graphite has (delocalized) mobile electrons.
Calculating Empirical Formula Process.
Percentage Given/Relative Atomic Mass.
Result/Smallest Ratio(Value)
Two Basic Differences between Boiling and Evaporation.
Boiling occurs at a specific temperature while Evaporation can occur at any temperature.
Boiling has bubbles.
Formula to Determine energy change in reaction,
[Energy Taken In] - [Energy Given Out]
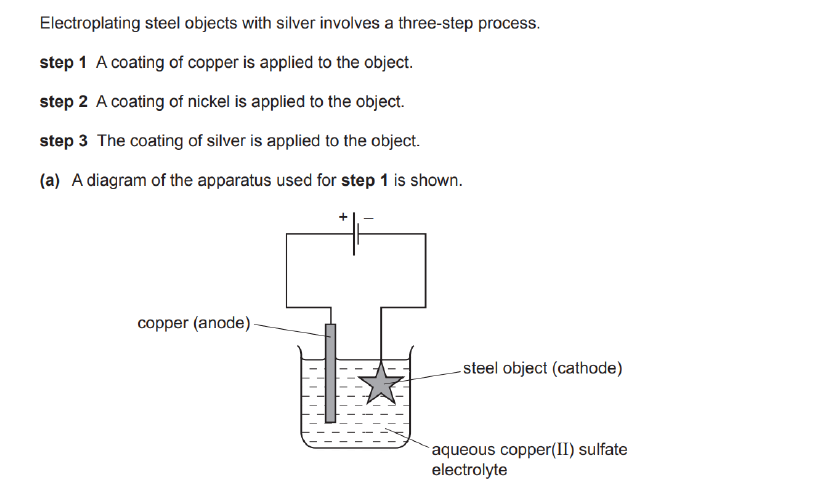
Explain why the concentration of copper ions in the electrolyte remains constant throughout step 1.
Rate at which Copper II ions removed at the cathode is equal to Cu²+ ions replaced at anode. (Equilibrium essentially.)
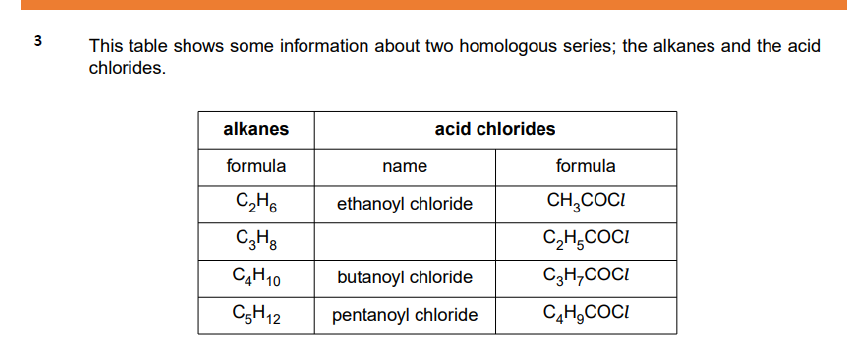
Would you expect the products of complete combustion of acid chlorides to be the same as an alkene? Explain your reasoning.
No, the products of complete combustion of acid chlorides differ from those of alkenes. Acid chlorides produce carbon dioxide and hydrochloric acid, whereas alkenes yield carbon dioxide and water.

Explain why the mixture initially turns darker brown.
Pushing in the plunger increases pressure, so the amount of brown NO₂ per volume increases, making the mixture appear darker.
How do you calculate energy change?
ΔH = Bonds broken − Bonds made.
Breaking bonds = requires energy → positive
Making bonds = releases energy → negative

State two observations that would show hydrochloric acid is a stronger acid than ethanoic acid.
The solid will dissolve faster in HCL.
Rapid formation of Bubbles.
Name the catalyst needed to form an ester from ethanoic acid and methanol.
CONCENTRATED Sulfuric Acid.
Percentage Yield Formula.
Actual Mass-Volume of gas collected/Expected Mass-Volume of Gas x 100.
Why is the speed of rate of reaction not constant in terms of volume accumulated per second?
The rate of reaction decreases over time as the concentration of reactants decreases, leading to fewer collisions between particles, until the limiting reactant is finished up.
Increase in Temperature increases rate of reaction, why?
Temperature Increase causes Kinetic Energy to Increase.
More particles have energy greater than or equal to activation energy
This increases the number of effective collisions.
Why does increasing the surface area increase the rate of reaction?
Increasing the surface area increases the rate of reaction by increasing the number of effective collisions, as more solid is exposed to collisions.
What causes Carbon monoxide to be produced in a car engine?
Carbon monoxide is produced due to incomplete combustion of fuel/diesel.