C4 & C5 chemistry
1/107
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
108 Terms
carbon dioxide test
add lime water (calcium hydroxide)
if present, cloudy and bubbling
CO2 reacts with lime water & forms insoluble carbonate
Chlorine gas test
tap water on blue litmus paper so damp
bleaches damp blue litmus paper turning it red (due to acidity) then white
Hydrogen test
lit splint over the open end of a test tube containing hydrogen gas
will create a squeaky pop noise
made because hydrogen burning rapidly with oxygen to make water
Oxygen test
glowing splint over a test tube containing oxygen gas
glowing splint inside a test tube will relight if oxygen is present
Flame test
when metal ions are heated, energy is transferred to their electrons
This makes the electrons become excited and move up to higher electron shells
at these higher energy levels, they are unstable and move back down to their normal electron shells
as they move back down, energy is transferred to the surroundings as radiation, which is seen as light
Different metal ions produce different colours
Flame colours for common metal ions
Lithium →red
Sodium → yellow
Potassium → lilac
Calcium → orange-red
Copper → green-blue
Group 1 metals
alkali metals
form alkaline solutions when reacted with water
Physical properties of Group 1
soft metals
increase in softness going down group
low densities
very reactive
good conductors of electricity
why do Group 1 metals have relatively low MPs decreasing going down group?
gets further away from nucleus- atoms get larger due to increasing number of shells, creating a greater space away from nucleus. this distance decreases attractive forces between outermost electron and nucleus’ positive charge
reactivity trend of Group 1
increases going down group (weaker FOA to overcome)
only one electron lost, so easier to lose electron
then obtains noble gas configuration
Alkali metal + oxygen
_____ oxide (superoxide for K)
Alkali metal + Chlorine
____ chloride + a white precipitate
Alkali metal + Water
____ Hydroxide + Hydrogen
Li + H2O observation
slow reaction
Li doesn’t melt
fizzing can be seen and heard
Na + H2O Observation
large amounts of heat causes Na to melt
Hydrogen catches fire
Na dashes on the surface
K + H2O Observation
reacts violently
enough heat released so hydrogen burns & produces a lilac flame
K melts into a shiny ball
K dashes on the surface
Why does reactivity increase going down the group?
Alkali metals only lose 1 electron to gain noble gas configuration
Going down, number of shells increase by 1
further away from nucleus= weaker forces of attraction
less energy required to overcome FOA so electron is lost more easily
Group 7
Halogens
diatomic elements which form -1 halide ions
formed by a single covalent bond
Group 7 State & Appearance at room temp
F - yellow gas
Cl - pale yellow/green gas
Br - red/brown liquid
I - purple/black solid
Characteristics of Group 7 elements
F- very reactive, poisonous gas
Cl- reactive, dense, poisonous gas
Br - dense red-brown volatile liquid
I - shimmery, crystalline solid, sublimes to form purple vapour
Boiling and Melting point trends
increase going down the group
intermolecular forces strengthen as atoms get larger, more energy required to overcome forces
reactivity trend in Group 7
decreases going down
fluorine- smallest halogen and closest to nucleus. ability to attract an electron is strongest in F2, making it most reactive
Halogen displacement reaction
when a more reactive halogen displaces a less reactive halogen from an aqueous solution
NEED TO KNOW: Cl, Br, I (most → least reactive)
Displacements of Cl, Br, I
Chlorine displaces: Br (yellow/orange colour seen), I (brown colour is seen)
Bromine displaces: I (brown colour is seen)
Iodine displaces: none
Chlorine + Bromine half equation
Cl2 + 2br- → 2Cl- + Br2
Chlorine + Iodine half equation
Cl2 + 2I- → 2Cl- + I2
Bromine + Iodine half equation
Br2 + 2I- → 2Br- + I2
Metal Halide reactions
Cl, Br, and I react with metals to form compounds
create metal halide salts
halides hold a -1 charge
rate of reaction is slower for halogens which are further down the group
Non metal halides
halides react with non metals to form simple molecular covalent structures
Group Zero
Noble gases
monatomic, colourless, non flammable gases at room temp
unreactive
Group 0 characteristics
low density
increasing density down group/ get heavier
non metals
uses of Group 0 elements
chemically inert
helium fills balloons as it doesnt burn and is less dense
Neon, argon & Xenon are used in signs
Ar is used to fill light bulbs
Ar creates inert atmosphere for welding
gases at room temp
individual atoms are widely spaced apart and so they have low densities
Why do group 0 have low MPs and BPs?
atoms get larger moving down group
BP increases going down (although still below 0*c)
increase in intermolecular forces increasing amount of energy needed to overcome these forces
Transition metals properties
hishly dense
good conductors of heat & electricity
lustrous
malleable
shiny when freshly cut
form coloured compounds
hard, strong metals
multiple oxidation states
use of transition metals
catalysts
they don’t take part in the reaction
catalytic ability stems from their ability to interchange oxidation states
can form complexes with reagents which can easily donate and accept electrons
COMMON METAL CATALYSTS*****
iron- HABER process
vanadium pentoxide- contact process
nickel- hydrogenation of alkenes
oxidation state facts
compounds containing transition metals in different oxidation states will have different properties & colours in aqueous solutions
PREDICTING REACTIVITY OF DIFFERENT GROUPS
Group 1-2: highly reactive- increasing reactivity, forms ionic compounds with NM
Group 7: get less reactive going down group
Group 0: elements are unreactive
Transition metals VS Group 1
G1- +1 ions, TM- ions with variable charges
G1- soft metals, TM- hard, strong metals
G1- low MPs, TM- much higher MPs
G1- very reactive, TM- less reactive
G1- reacts with O2,H2, and G7, TM- react slow or not at all
G1- tarnish in presence of oxygen, TM- takes several weeks to form metal oxides & require water
Reactivity series facts
most metals react with dilute acids like HCl
metal atoms form positive ions by loss of e- when reacted
tendency of a metal to lose e- is measure of its reactivity → more reactive= easier it is to lose electrons
metals that react with cold water form metal hydroxide + hydrogen
Metal + Acid…
→ metal salt + hydrogen
iron + hydrochloric acid → iron(II)chloride + hydrogen
METAL BECOMES A POSITIVE ION
reactivity series in order
K (reacts violently with H2O and acid)
Na (reacts quickly with H2O, violent w/ acid)
Ca
Mg
Zn
Fe
H
Cu
Ag
Au
rate of H2 production
more reactive a metal is = greater rate of H2 production so reaction is more vigorous
When do metal + acid/ water reactions take place?
if the metal is able to displace the hydrogen in them
Metal Cations
can be identified by the colour of the precipitate they form on addition of NaOH
PRACTICAL
few drops of NaOH slowly
Metal Cation colours
Iron (II): green
Iron (III): orange-brown
Copper (II): blue
Calcium: white
Zinc: white
Aluminium: white
Magnesium: white
How to differentiate the metal cations which create white precipitates?
add NaOH in excess
Zinc and Magnesium will dissolve into colourless solutions. Calcium and Aluminium won’t.
They can also be Flame tested to establish identity.
Test for Carbonate ions
add dilute acid
if carbon is present, CO2 will be formed, bubbles will be seen.
Add gas with limewater, Ca(OH)2, and IF CARBONATE PRESENT: white precipitate and CaCO3 will be formed.
Sulfate ion test
acidify sample with dilute HCl
add barium chloride (or nitrate)
IF SULFATE PRESENT:
white precipitate is formed
Halide testing
acidify sample with dilute nitric acid (HNO3)
this removes carbonate ions which may give a false positive
add silver nitrate (AgNO3)
IF PRESENT: Silver halide precipitate is formed (AgX)
Flame Testing
dip loop of unreactive metal in dilute acid, and hold it in blue flame until there is no colour change (sterilisation step)
Dip loop into solid sample
Place loop at the edge of bunsen blue flame
colour can be observed
Flame Test Colours
Li: Red
Na: Yellow
K: Lilac
Ca: Orange/red
Cu: Blue/green
Flame emission spectrometer
detailed analysis
used to identify multiple ions present
How does a Flame Spectrometer work
exposes sample to a very hot flame and then measuring the intensity and wavelength of light emitted
image created is viewed as a line emission spectra and each element has a characteristic pattern
What can affect rate of reaction?
concentration of reactants in solution
pressure
temperature
surface area
catalyst
economic interest
higher rate of reaction
high atom economy
high percentage yield
Effect of increase concentration or pressure
increases rate of reaction
on a graph, line will be steeper.
Effects of increasing surface area and temperature
same effect as concentration and pressure.
Collision theory
chemical reactions only occur when reactant particles collide with sufficient energy to react
rate of reaction is dependent on the energy and numbers of the collisions
Why does increasing concentration or pressure of a solution increase rate of reaction?
more reactant particles in a given volume- more frequent and successful collisions per second
Why does an increase in temperature increase rate of reaction
particles have more kinetic energy than required activation energy
more successful and frequent collisions per second
Why does a larger surface area increase rate of reaction
more room for reaction to take place so higher rate of reaction
Haber process
catalyst of iron
production of ammonia
450 degrees
200 atm
Sulphuric acid Contact process
detergents, paints, fibres
450 degrees
2atm
vanadium pentoxide
Calibration curve
light intensity produced is directly proportional to number of ions vapourised
used to determine concentration of metal ions in a solution by reference to a standard solution of known concentration on a calibration curve
mass spectrometer
powerful analytic technique. The most useful instrument for accurate determination of the relative atomic mass of an element based on the abundance & mass of each of its isotopes
also used to find the relative molecular mass of molecules
a spectrum is produced of the mass/charge ratio against abundance
There are several types, but all are based on the ratio of their charge to their mass
Mass spectrometry in identifying isotopes
The height of the peaks shows the proportion of each isotope present
molecules- not just atoms- can be analysed
The molecular ion peak can identify molecular mass of a compound, however, different compounds may have the same molecular mass
molecules can fragment as they are ionised and the fragments can pass through to give a range of different peaks- can fragment due to formation of characteristic fragments, or loss of small molecules
Advantages of instrumental analysis
-can analyse chemical substances due to advancements in technology
X-rays, Infra-red, Mass Spectroscopy, Gas Chromatography, Flame photometry
-provide greater accuracy
-faster and easier to use
-automated and can perform multiple simultaneous sampling and testing
-very sensitive and can work with very small sample sizes
concentration in g/dm³
mass of solute (g) / volume of solution (dm³)
concentration
measure of how much of a substance is present in a given volume
converting between g/dm³ and mol/dm³
from g/dm³ → to mol/dm³ = DIVIDE BY MOLAR MASS IN GRAMS
from mol/dm³ → to g/dm³ = MULTIPLY BY MOLAR MASS IN GRAMS
cm³ to dm³
divide by 1000
dm³ to cm³
multiply by 1000
concentration in mol/dm³
moles per unit volume
concentration = moles / vol(dm³)
titration
analysing the concentration of a solution
acid base titrations are commonly used to determine exactly how much alkali is needed to neutralise a quantity of acid
used to prepare salts or other precipitates in redox reactions
indicators show the end point in a titration
PHENOLPHTHALEIN being a popular choice
indicator choice in titrations
PHENOPHTHALEIN is a popular choice- distinct colour (pink) shows
wide range indicators, like litmus, aren’t suitable as they don’t give a sharp enough colour change at the end point
universal indicator isn’t suitable- mix of indicators and has too many subtle colour changes
health and safety in titrations
dilute HCL- may cause harm to eyes or skin
Acids & alkalis are corrosive and should be handled with care
avoid contact with the skin & use safety goggles with both substances
pipette should always be used with a safety filler to avoid contact
EQUIPMENT in titrations
25cm³ volumetic pipette
pipette filler
50cm³ burette
250cm³ conical flask
small funnel
0.1 mol/dm³ NaOH solution
sulphuric acid- concentration unknown
phenolphthalein indicator
clamp stand, clamp & white tile
titration practical
use pipette to place exactly 25cm³ NaOH solution into conical flask
place conical flask on a white tile soo tip of burette is inside of flask
add a few drops of a suitable indicator to the solution in conical flask
perform rough titre by taking burette reading and running solution in 1-3 portions while swirling flask continually
close tap when colour change is reached, and record volume, placing eye level with meniscus
now repeat with fresh NaOH
as rough end point volume is approached, add solution from burette one drop at a time until indicator changes colour
swirl after each addition and rinse the sides of the flask down with distilled water to make sure that all that was added was reacted
finish at first sign of colour change & record volume to nearest 0.05
repeat until 2 concordant results
REMOVE FUNNEL AFTER FILLING BURETTE- CAN DROP SOLUTION INTO BURETTE- LEADING TO ERROR
GAS VOLUME CALCULATIONS
volume = amount of gas (g) x 24 dm³mol^-1
avogardro’s law
at the same conditions of temp and pressure, equal amounts of gases occupy the same volume of space.
at RTP and pressure, volume CCEPTED BY 1 MOLE OF ANY GAS is found to be 24dm³.
20 degrees celcius, 1 ATM
reaction yield
(actual / theoretical) x 100
reaction yield explained
amount of products retrieved from a reaction- you never get 100% yield in a chemical process for several reasons.
reasons in theoretical yield
reactants left behind
reactions may be reversible
a high yield is never possible
products may be lost during separation and purification
may be side reactions - gas, precipitates
can be lost in transfer of containers
actual yield & theoretical yield
actual: recorded amount
theoretical: amount obtained under perfect practical & chemical conditions
percentage yield compares the two
economics of yield
businesses look at yield to check out how successful chemical processes are, and will try it out with different reaction pathways, which are compared and evaluated, so a manufacturing process can be chosen
- companies look for a high percentage yield as possible to increase profits and reduce costs and waste- COST EFFICIENT
atom economy
analyses the efficiency of reactions
studies the amount of reactants that get turned into useful products- illustrates what percentage mass is turned to useful product, and is used to obtain sustainable development
atom economy formula
100 x (total Mr of desired product / total Mr of all products)
choosing a reaction pathway
reactions which have low atom economy use up lots of resources and produce a lot of waste material, which then needs to be dispose of, which is very expensive. UNSUSTAINABLE AND NOT ECONOMICALLY ATTRACTIVE.
companies AE
companies analyse different reaction pathsways to improve efficiency.
atom economy, percentage yield, and efficiency are important and need to be considered when choosing a reaction pathway. High Yield & fast rate of reaction are desirable
in reversible reactions, position of equillibrium may need to be changed in favour of the products by altering reaction conditions
measuring rates
reactant used(or product formed) = rate of reaction x time taken
reaction times
different reactions take place at different rates: rusting= slow, explosions= fast
rate of reaction
rate of reactions can be measured either by how fast a reactant is used up, or by how fast the product is made
product made equation
product made = rate of reaction x time taken
measuring rates units
cm³, dm³, or volume.
usually measuring time in seconds
measuring mass
if product is a gas→ reaction can be performed in an open flask on a scale to measure loss in mass of reactant.
-cotton wool is placed in the mouth of the flask, which allows gas out, but prevents any molecules from being ejected- not good for H2 or any gas with a small Mr
measuring volume of gas
gas trapped and volume is measured in pushed out gas syringe.
(do the one tub one if gas is not water soluble)
exampls: Mg + HCl → H2 + MgCl
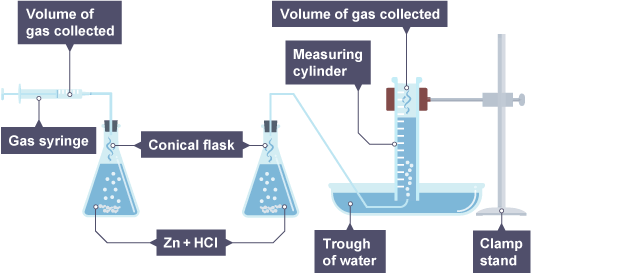
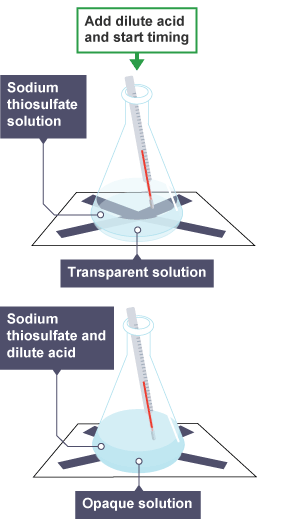
measure of precipitates
*for example, sodium thiosulfate + hydrochloric acid (Na2S2O3 + 2HCl)*
time as you add acid
watch until you cannot see X
rate graphs
useful for calculating mean rate of reaction
rate at specific point
time a reaction until it reaches completion
→ reactants will decrease, as concentration of products increase