other functional groups
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
A carbonyl compound is a compound that contains...
C=O
general formula of aldehydes?
CnH2nO
We call a molecule an aldehyde when it contains a carbonyl group bonded to…
a carbon and a hydrogen/two hydrogens
How do we write the structural formula of aldehydes?
RCHO
What’s the name of this molecule?
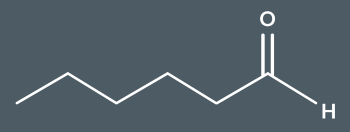
hexanal
What’s the name of this molecule?
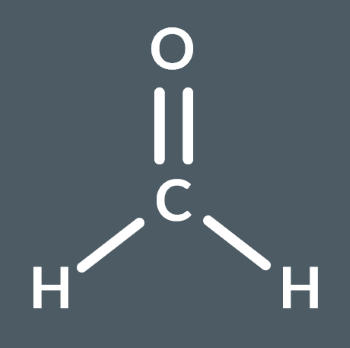
methanal
What is the general formula of ketones?
CnH2nO
ketone
contains a carbonyl group bonded to two carbons
The general formula of ketones is the same as the general formula of...
aldehydes
How do we write the structural formula of ketone?
RCOR
What’s the name of this ketone?
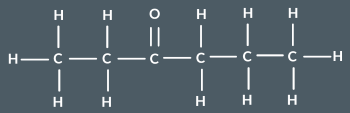
hexan-3-one
When naming ketones, we write the position number...
before the ‘one’
general formula of carboxylic acids?
CnH2n+1COOH
The general formula of a carboxylic acid with 3 carbons is...
C2H5COOH
What’s the name of this molecule?
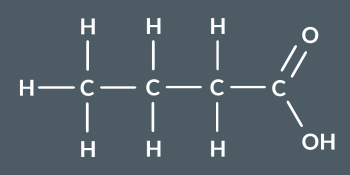
butanoic acid
We call a molecule a carboxylic acid when it contains a carbonyl group that’s bonded to…
a hydrogen and a hydroxyl group
a carbon and a hydroxyl group
Aldehydes, ketones and carboxylic acids all experience which forces…
Van der Waals forces.
dipole-dipole forces
Hydrogen bonding only occurs when hydrogen is covalently bonded to…
fluorine, oxygen, nitrogen
For carbonyl compounds with the same number of carbons, we would expect the melting and boiling points of…(3)
aldehydes and ketones to be similar
carboxylic acids to be higher than aldehydes
carboxylic acids to be higher than ketones
As the length of the carbon chain in carbonyl compounds increases, the...(3)
strength of Van der Waals forces increases.
melting point increases
boiling point increases.
Because carbonyl compounds contain a polar bond, we would expect solubillity to...(2)
small carbonyl compounds to be highly soluble in water.
their solubility to decrease as the length of the carbon chain increases
carboxylic acids have a hydroxyl group. Compare solubility to other carbonyls
more soluble than aldehydes
more soluble than ketones
The most soluble carbonyl compound is...
carboxylic acids
Predict which of these compounds has the highest boiling point:
Propanoic acid
Butanone
Propanal
Propanoic acid
Propanoic acid has a higher boiling point than butanone and propanal because propanoic acid experiences hydrogen bonds between the hydrogen and oxygen atoms of its hydroxyl groups. Hydrogen bonds are the strongest of the intermolecular forces ((Van der Waals forces << dipole-dipole forces << hydrogen bonds).).
Predict which of these compounds is most soluble in water:
Propanal
Propanone
Propanoic acid
Propanoic acid
All of these small carbonyl compounds are highly soluble in water because they contain a polar carbonyl group that can form hydrogen bonds with water molecules.
However, unlike propanal and propanone, propanoic acid also has a hydroxyl group. This means that propanoic acid can form more hydrogen bonds with water molecules, making it most soluble in water.