biochem polysaccharides slides
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
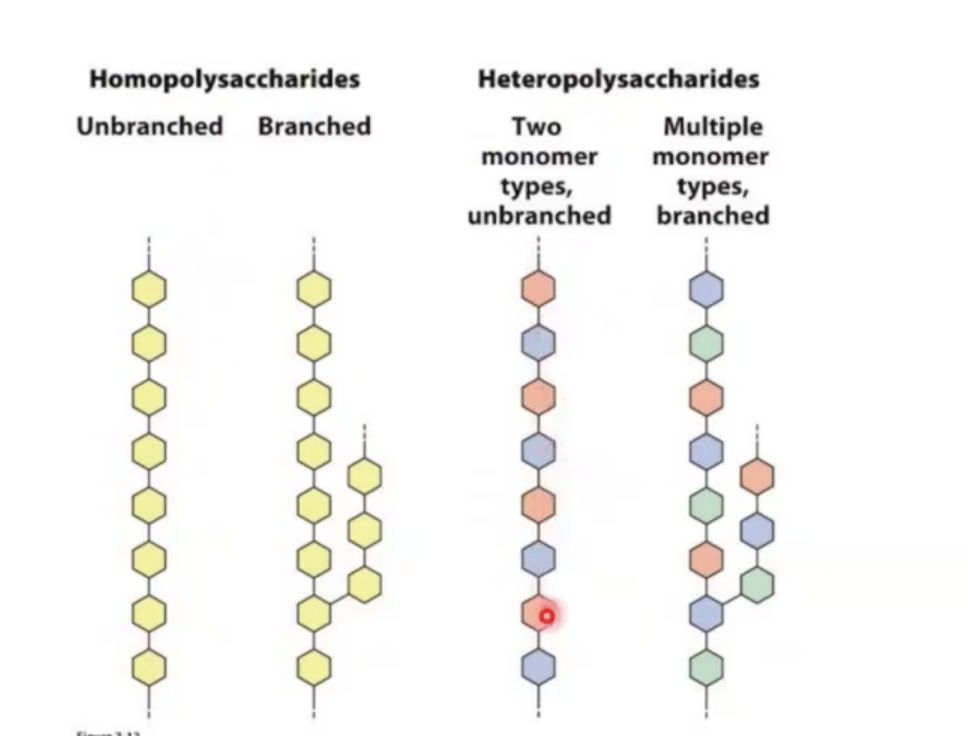
homopolysaccharide vs heteropolysaccharide
homo: same sugar repeating
hetero: different sugars repeating
hexokinase is useful for?
glucokinase is useful for?
muscle tissue.
liver tissue (glucokinase is very important for storing sugars)
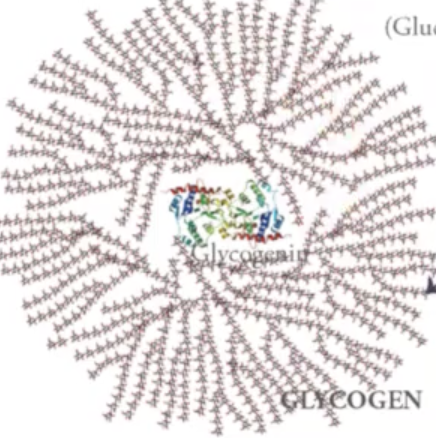
glycogen
is a large polysaccharide with branched structures around it which are α 1, 4 linked or α 1, 6 linked sugars.
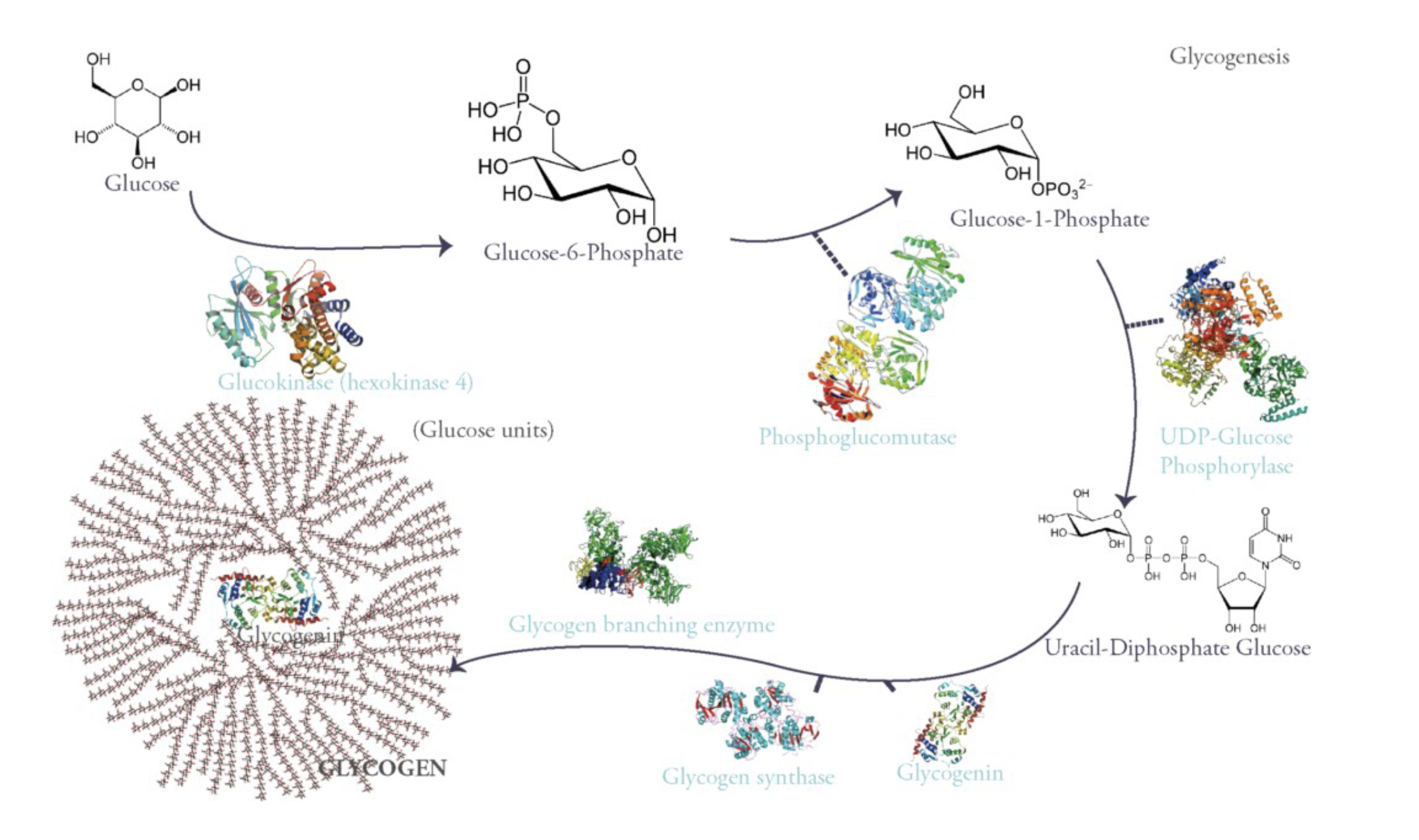
to generate glucose:
first you have a glucose kinase enzyme that is going to phosphorylate glucose in the 6th position which gives you glucose-6-phosphate. This traps glucose in liver cells.
Once glucose-6-phosphate is formed, it could go into glycolysis but in liver cells what is happening is that you trapped glucose out of the bloodstream inside the cells so that lowers blood glucose levels.
Then there is a phosphoglucomutase (moves functional group from one place to another on the same structure. in this case it moves phosphate group on glucose-6-phosphate from the 6th position to position 1.)
once that happens, UDP-Glucose phoshorylase enzyme will link that glucose-1-phosphate to a uridine monophosphate to give uracil-diphosphate glucose (UDG). This serves as an activated substrate for assembling this large glycogen structure using glycogen synthase and glycogen branching enzyme.
This glycogenin protein is packaged along with glycogen (glycogen is responsible for cleaving some of those glucoses in case you need to raise levels of glucose in blood.
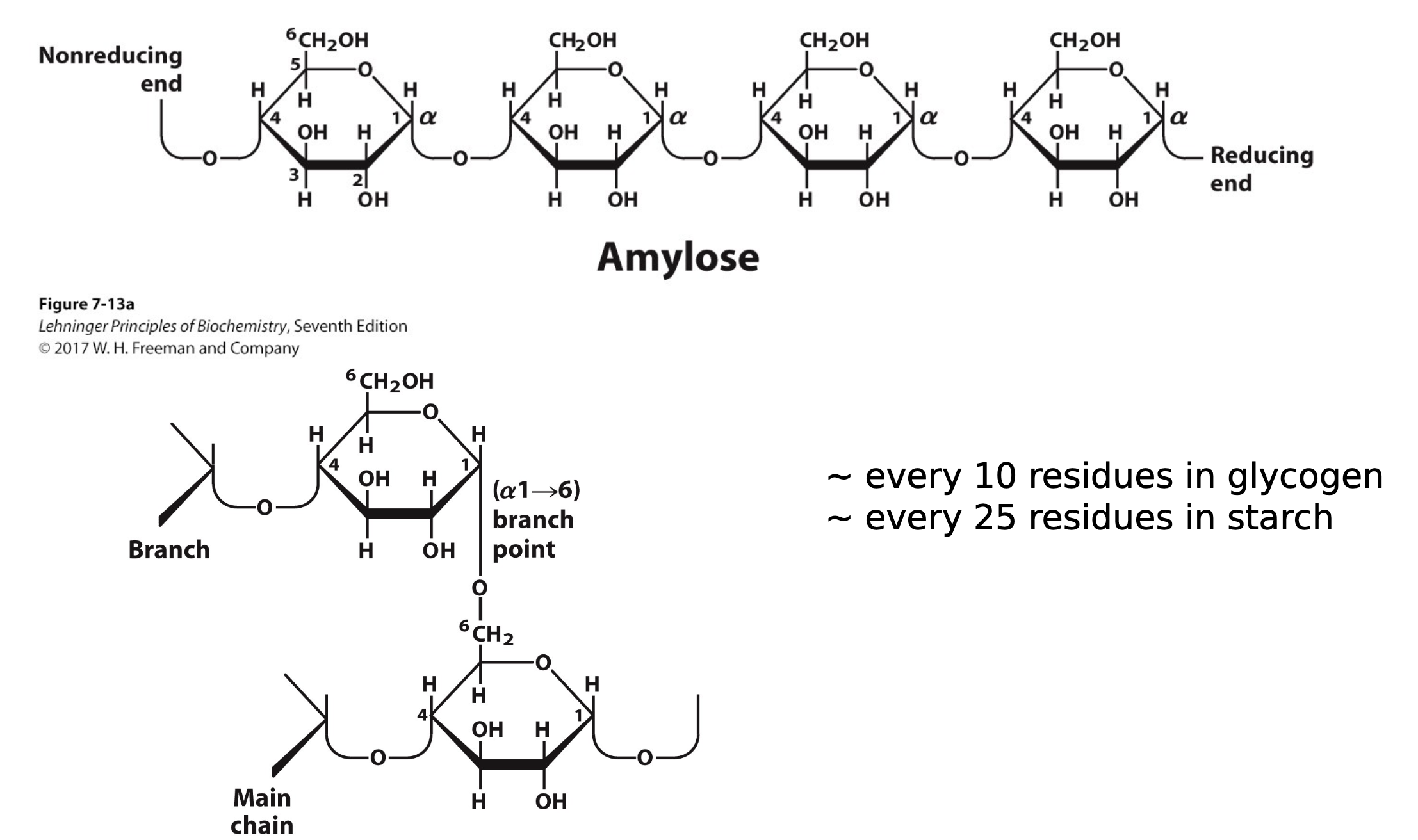
glycogen is made up of what polysaccharide?
for every 10 residues, what happens?
but in plants it is for every __ residues.
amylose type of linear polysaccharide which is just glucose1-4 linked to give you a larger structure. every 10 residues after these glucose monomers, you will have a branch from the 6th position of one branch to the one position of the next branch and then you will build another amylose polymer off of that.
25 residues.
this is a α 1-4 linked polysaccharide of glucose which is glycogen or starch.
recall that there is an enzyme that is responsible for breaking down α 1-4 linked sugars, what is the importance of this?
recall that there is an enzyme that is responsible for breaking down α 1-4 linked sugars, so that is how we get energy back out of those sugars. we can cleave them if we need them.
there is a β1-4 linked sugar that behaves differently for humans. this β1-4 linked polysaccharide is called?
what purpose does it have?
cellulose. cellulose forms these really tight hydrogen bonds between polymers of this molecule also in between the individual sugars that make up this glucose chain. what makes cellulose insoluble in water is this hydrogen bonding. water cannot fit in between these strands, so now you have an insoluble polymer that is a very polar polymer but it is still insoluble in water.
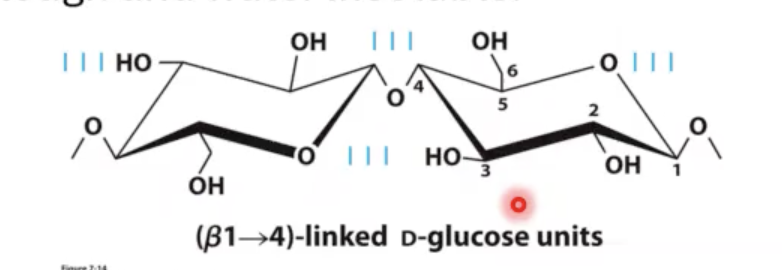
why are animals able to eat grass but not us?
animals have a separate organ that is responsible for holding onto many different microorganisms that can actually digest these β1-4 linked sugars. this enzyme is called cellulase. they are only produce by certain types of fungi, bacteria, and protozoa.
who produces cellulase enzymes?
ruminants and termites live symbiotically with host organism and they produce these cellulase enzymes that allow certain animals (like cows) to gain nutrition off of these types of sugars.
so name some homopolymers of glucose:
(glycogen and starch) and cellulose
name some heteropolymers:
Glycosaminoglycans
Glycosaminoglycans
always have a repeating disaccharide structure where one of the sugars associated with that disaccharide is either N-acetylglucosamine or N-acetyl galactosamine or even derivatives of those.
they either have uronic acid (sugar that has been oxidized at C6 position to the carboxylic acid) or it will have sulfate esters.
they can form large meshworks with fibrous proteins (like collagen and elastin, etc.) that make the extracellular matrix of connective tissue and promote lubrication of joints.
Heparin
is a Glycosaminoglycan. it is a linear polymer (3-40 kDaltons) of Ido uronic acid [(L sugar arrangement) that has been sulfated at the 2nd position and at the 6th position it has been oxidized.] and glucoseamine that instead of an acetyl group at position 2 there is a sulfate and at the 3rd position there is a sulfated hydroxyl group there and at the 6th position there is another sulfate.
This is repeated over and over again to give heparin molecule.
![<ul><li><p>is a <span>Glycosaminoglycan. it is a linear polymer (3-40 kDaltons) of Ido uronic acid [(L sugar arrangement) that has been sulfated at the 2nd position and at the 6th position it has been oxidized.] and glucoseamine that instead of an acetyl group at position 2 there is a sulfate and at the 3rd position there is a sulfated hydroxyl group there and at the 6th position there is another sulfate.</span></p></li></ul><p></p><ul><li><p><span>This is repeated over and over again to give heparin molecule. </span></p></li></ul><p></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ee660920-ea92-4fcf-b8eb-33d8bb838538.png)
heparin vs heparin-sulfate
same sugar structure but heparin-sulfate is attached to proteins.
heparin has highest __ out of other biomolecules.
Highest negative-charge density (so they are highly negatively charged)
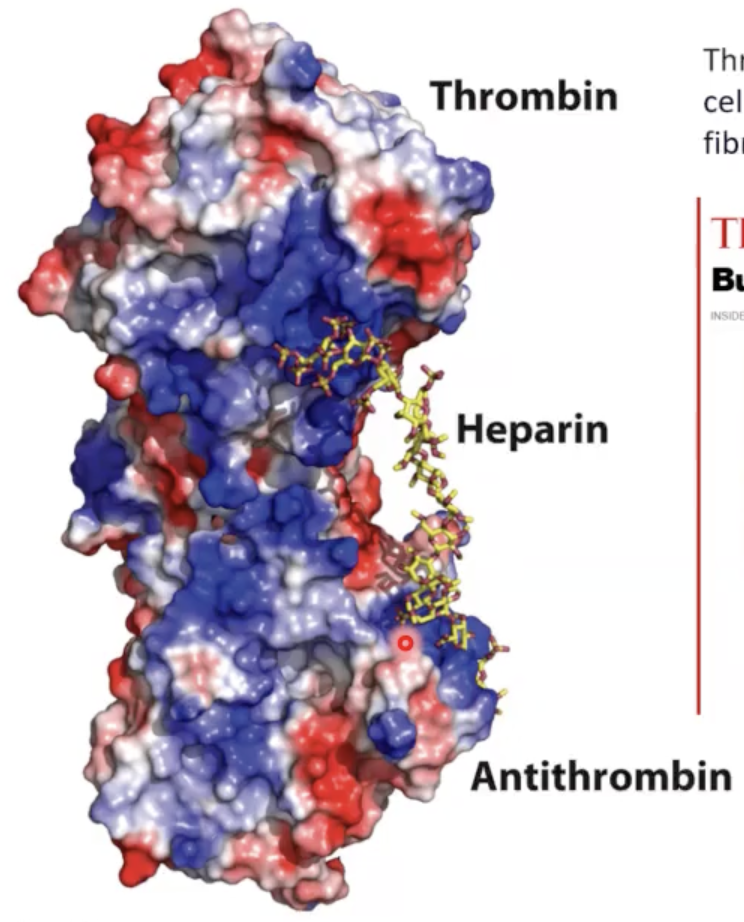
heparin functional roles?
• Prevent blood clotting by activating protease inhibitor antithrombin [thrombin does blood clotting and antithrombin stops blood clotting].
• Binding to various cells regulates development and formation of blood vessels.
• Can also bind to viruses and bacteria and decrease their virulence.
Thrombin clots blood by activating __ and chopping up a protein called ? to form ?.
platelets cells.
fibrinogen.
fibrin.
In the year 2008, there was a contaminated __ that was given out from china which caused many deaths/injured numbers. you do not want blood to clot while surgery is happening.
heparin
when thrombin becomes activated to promote cleavage of fibrinogen protein, it has to move away from __.
these blue patches means __. since heparin is negative, it associates with these positive blue residues of thrombin and antithrombin that essentially locks these two proteins together.
usually thrombin is in its inactive state, when there is a signal that requires blood clotting, heparin will separate from this which leads to thrombin separating from antithrombin. this allows thrombin to begin blood clotting. we increase amount of __ prior to surgery to prevent thrombin from doing its job.
antithrombin (protease inhibitor).
positively charged amino acid residues in protein.
heparin.
we get heparin from?
the china company was running out of pigs so they decided to cut heparin that they produced with other material that they had more of. this new material is a polysaccharide called __.
This Anti-arthritic medication is isolated from mammalian and fish cartilage.
pigs.
Chondroitin 4 -sulfate.
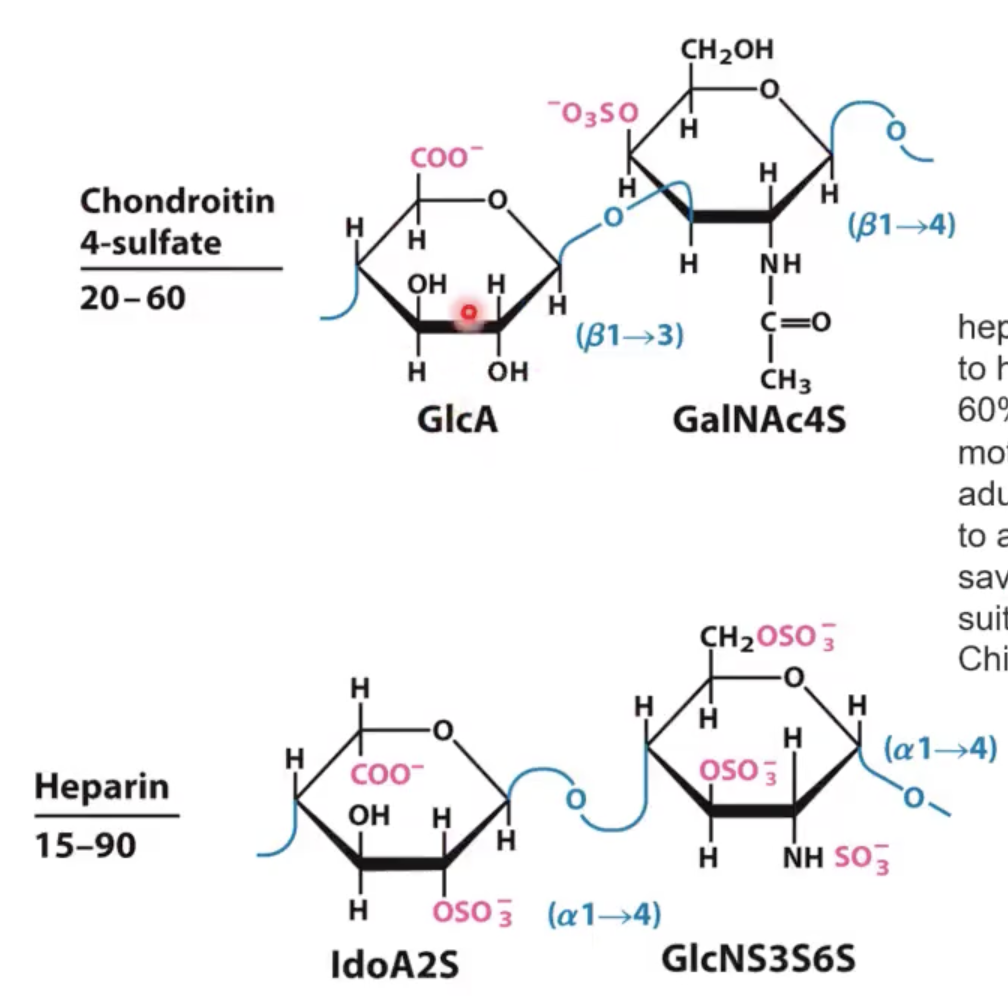
draw heparin vs chondroitin 4-sulfate (what is the difference?)
was using chondroitin 4-sulfate instead of heparin successful?
Instead of ido uronic acid, it has glc uronic acid.
Instead of (α1—>4) linked N-acetyl glucoseamine, you have a (β1—>3) linked N-acetyl glycoseamine with a sulfate at position 4.
NOPE!!!
why is it difficult to get FDA approval of new preps of heparin?
because these new preps of heparin are a little bit too clean (too pure heparin).
however if new drug prep is identical analytically to previous prep of that drug than you do not have to do a new drug application (this speeds up approval process). however since heparin comes from pigs, it always had a little contamination of something (not clear what that contamination is), so if you do not produce new heparin prep exactly like that then you end up having to do new drug approval which costs a lot of money.
t/f: we can use complex sugar structures to modify lipids and proteins.
these complex sugar structures are called?
TRUE.
glycoconjugates.
conjugate in glycoconjugates means?
what does glycoprotein mean?
you have covalently attached these glyco (sugar) units on to a protein or lipid.
ex: glycoprotein has to do with sugar modifying a protein. typically these proteins are modified with small sets of oligosaccarides. it can be a simple glcnac residue which is often the same location you get phosphate modification. you can get 8,9,10 sugar units attached to proteins.
about __ of mammalian proteins are glycoproteins.
only some bacteria __ some of their proteins.
much more prevalent modification in __ than it is in the rest of the kingdoms.
half.
glycosylate [the process of attaching sugar molecules (glycans) to a protein, creating a glycoprotein].
animals or in mammals
in vivo vs in vetro
in vivo: in organism
in vetro: done outside the body (like a testtube or dish)
very often if we are looking at a mammalian protein, it should be?
however bacteria, like ecoli, do not?
glycosylated.
glycosylate proteins all that much and when they do they do not glycosylate proteins with the same sugar units that mammals do.
the problem with using insect cells to look at glycoproteins is?
the problem with using mammal cells to look at glycoproteins is?
that the glycosylation pattern would not be the same as in mammals.
it is difficult to get a lot of protein to work out of mammalian cells.
these carbs (sugars) that are attached to proteins play a role in?
protein-protein recognition.
some viral proteins get heavily glycosylated which helps them evade immune system responses.
glycosylation of proteins comes in “two flavors”:
o-linked glycosylation.
n-linked glycosylation.
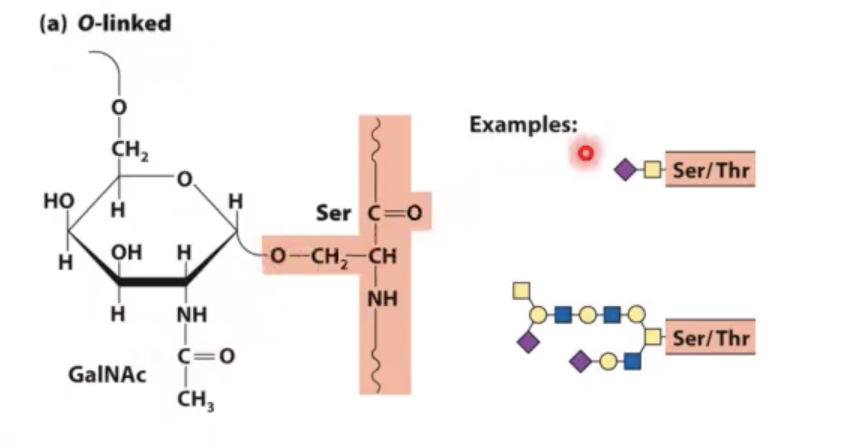
o-linked glycosylation.
you add a sugar unit on to a serine or threonine residue. you add through the oxygen of serine or threonine.
tends to happen in golgi apparatus in eukaryotic cells.
n-linked glycosylation.
modification of nitrogen of asparagine. this amide (NH) can be made nucleophilic enough to where we can attach these complex oligosaccharides on to this particular amino acid.
is a co-translational modification in eukaryotes where initially as proteins are coming off of ribosome in the endoplasmic reticulum there is an oligosaccharide transferase (OST) that transfers entire sugar block on to the appropriate asparagine residue in that protein as it is being formed. once that occurs, protein folds and goes into golgi apparatus to be shipped to final destination.
what happens in the golgi apparatus is that some of the sugars are trimmed off and new sugars are added on to give you various sugar structures that will be on the protein.
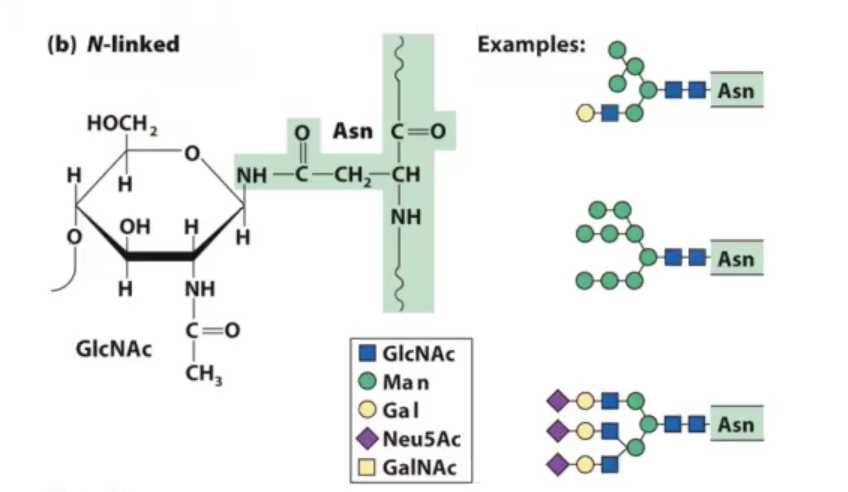
what we have noticed as proteins come out of the golgi apparatus is?
often have different levels of these types of modifications. many heterogenous structures because the machinery itself that is necessary to glycosylate proteins are not enough in number to keep up with all the proteins that are making their way through the golgi apparatus.
so we have mixed types of glycans on the surface of these proteins.
are mucin proteins typically highly glycosylated? they have sugar residues called?
name an example:
yes!
sialic acids (Neu5Ac).
MUC1 protein.
often our cells produce proteins that have this MUC1 protein which is highly __.
When this MUC1 protein is associated with cancer cells (because these cancer cells grow and divide so fast), the glycan that is put on this surface is __.
normally MUC1 would be completely covered with sugary units where you can barely access ? chain structure.
When you underglycosylate, some of the amino acid chains become __. they selected an antibody that would selectively interact with only the underglycosylated protein.
sialic.
under-glycosylated.
amino acid.
acessible
another place where these glycosylated proteins come into play are these?
name examples:
large proteoglycan aggregates.
Hyaluronan and aggrecan.
Hyaluronan forms __ (how big?).
huge (Mr > 2 • 10^8) noncovalent aggregates.
t/f: Hyaluronan and aggrecan are able to hold lots of water (1000x its weight) and provide lubrication to joints.
TRUE
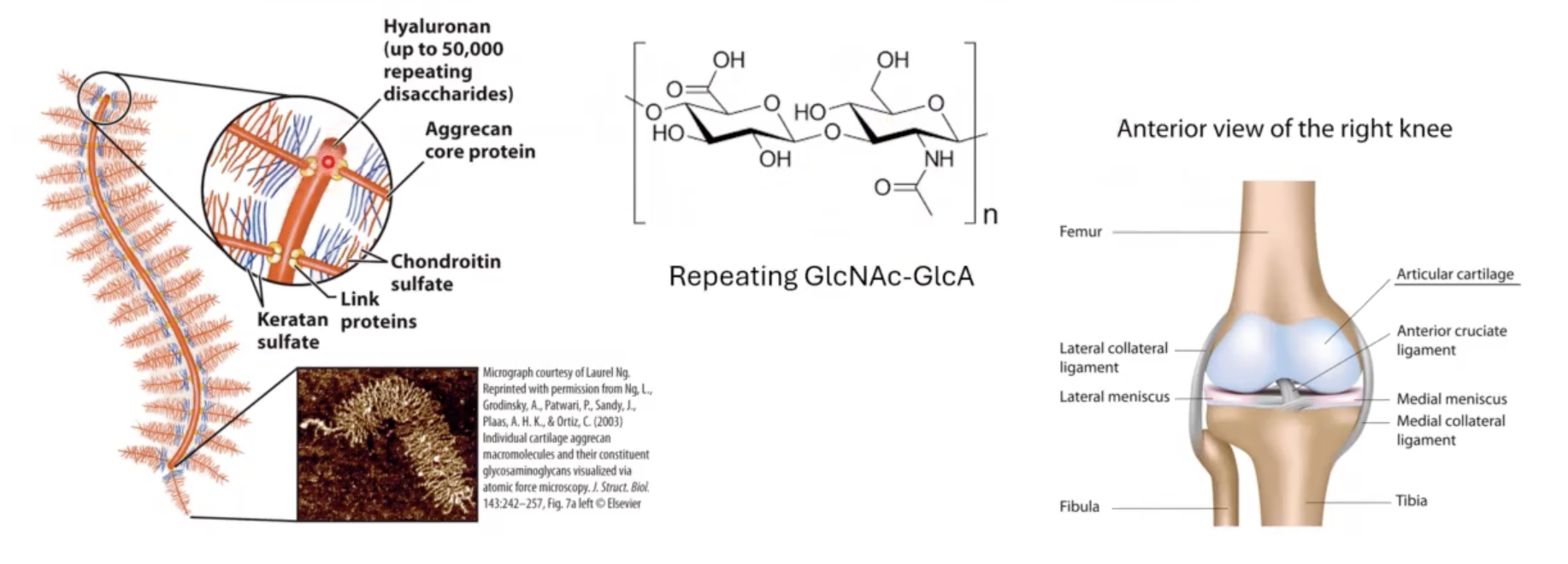
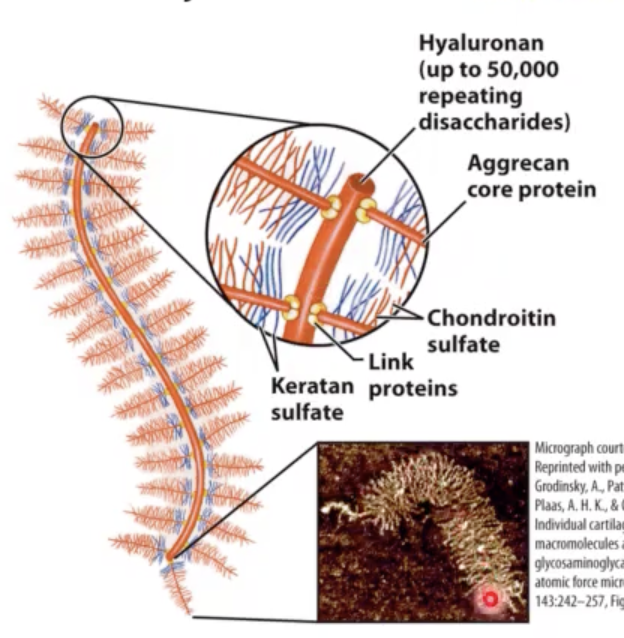
this disaccharide linked to?
other proteins (these other proteins are also heavily glycosylated) so we end up with bottle-brush like structures associated with Hyaluronan biomolecule.
because Hyaluronan holds so much water it is associated with different types of __.
for example when you squeeze your nose, you are squeezing out all the water from this Hyaluronan molecule but when you let go the water comes rushing in and retakes the original shape of nose.
cartilage.
outside of cell/tissues is made up of?
Extracellular Matrix (ECM).
Extracellular Matrix (ECM)
important for producing physical barriers and often are made up of large proteoglycan aggregates that will also include collagen fibers, proteoglycan aggregates, elastin (fibrous protein).
even some tumor cells will excrete certain types of __ like heparinases that degrade ECM to make it easier for these tumors to invade new tissue.
enzymes
besides glycoproteins what is another glycoconjugate?
glycolipids
glycolipids
when you have oligosaccharides directly linked to lipids.
ganglioside
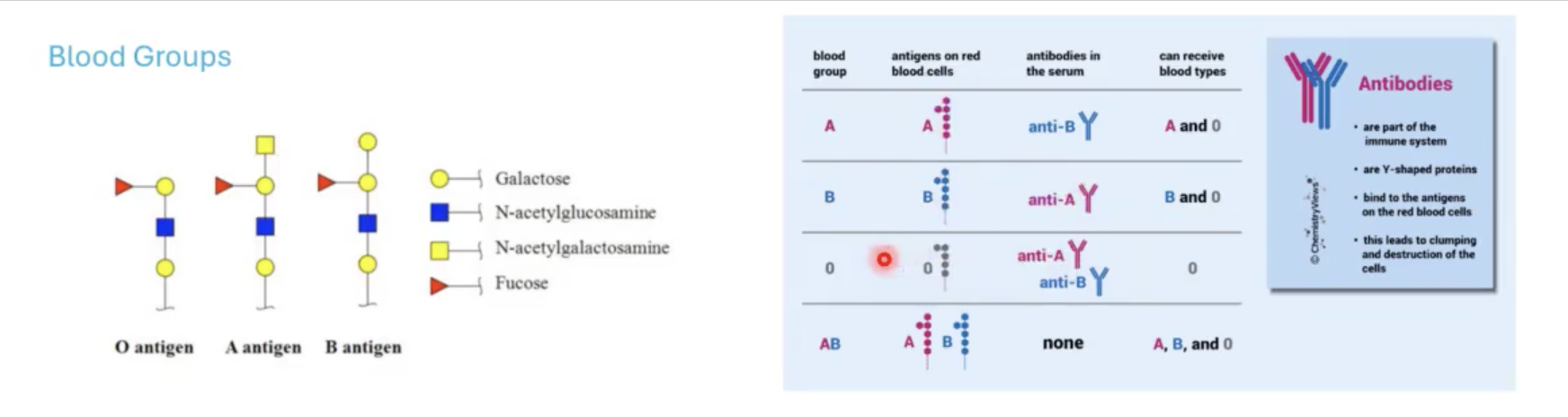
important for identifying the surfaces of different cells and determining blood groups.
blood groups are based on?
glycan antigens that are associated with surface of RBCs.
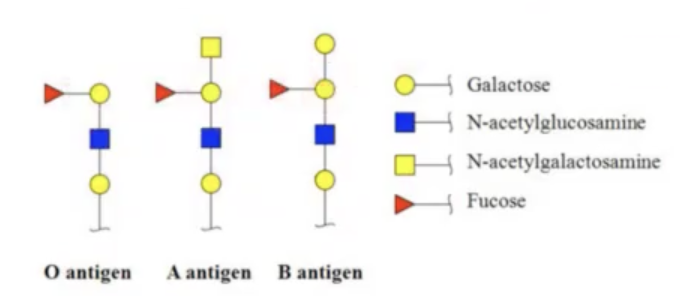
what blood is considered a universal donor?
O blood because it is a precursor for A and B.
Anyone with A and B blood will have the enzymes available to glycosylate this O antigen if you introduce this blood that is associated with O antigen.
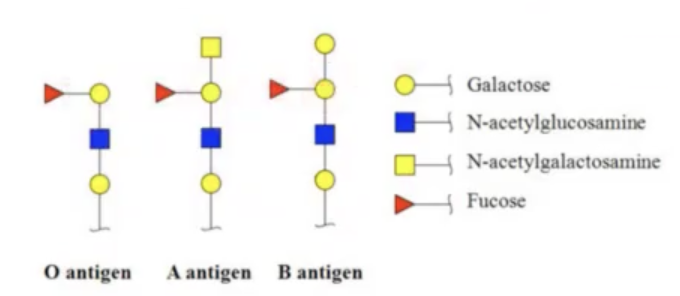
the reason why you cannot mix blood groups is?
for example for people with blood group A, they have developed anti-B antibodies (antibodies that will attack blood that has B antigen).
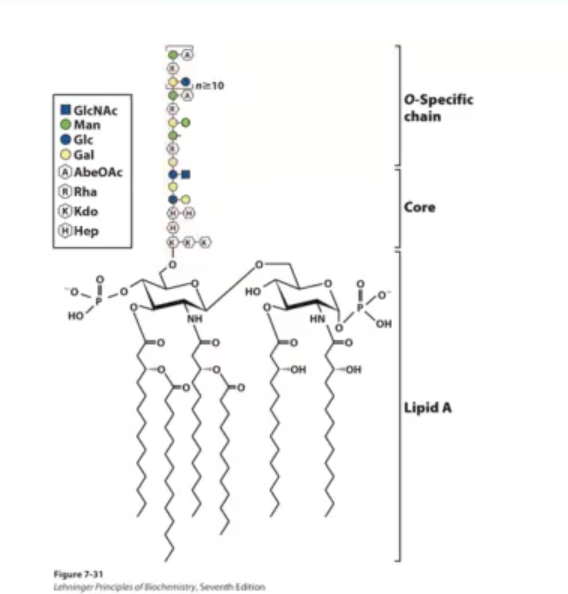
Bacterial Lipopolysaccharides
gram-negative bacteria has inner and outer membrane. the outer membrane is asymmetric where on the outside of the cell there are Lipopolysaccharides. Lipopolysaccharides have hydrocarbon chains that embed this larger structure into the outer leaflet of the outer membrane [that structure refers to Lipid A].
lipid A is a common structure found in many bacteria.
There are additional sugars linked to lipid A which are the Core and the O-specific chain. These complex sugars can often hide lipid A from immune system.
The picture shows E.coli LPS.
![<p>gram-negative bacteria has inner and outer membrane. the outer membrane is asymmetric where on the outside of the cell there are <span>Lipopolysaccharides. Lipopolysaccharides have hydrocarbon chains that embed this larger structure into the outer leaflet of the outer membrane [that structure refers to Lipid A].</span></p><p></p><p>lipid A is a common structure found in many bacteria.</p><p></p><p>There are additional sugars linked to lipid A which are the Core and the O-specific chain. These complex sugars can often hide lipid A from immune system.</p><p></p><p>The picture shows E.coli LPS.</p>](https://knowt-user-attachments.s3.amazonaws.com/359f8116-9104-448f-bbb3-2b6f3375b5d0.png)
toxic shock syndrome
happens after bacterial infection. it comes from our innate immune system being able to recognize lipid A as a foreign invader. it triggers a large immune response whenever lipid A is found in the bloodstream. because the immune response is so strong it can end up killing the person infected with toxic shock syndrome.
t/f: prokaryotes because of the crazy conditions they are able to live in, have very complex and weird sugar structures.
TRUE
the proteins sticking out of the influenza virus are called?
hemagglutinin (HA protein) (shorter ones) and neuraminadase (taller ones)
the influenza virus recognizes certain __ on the surface of cells (primarily respiratory cells). this sialic acid plays a role in acting as a ? for the influenza virus.
sialic acids.
receptor.
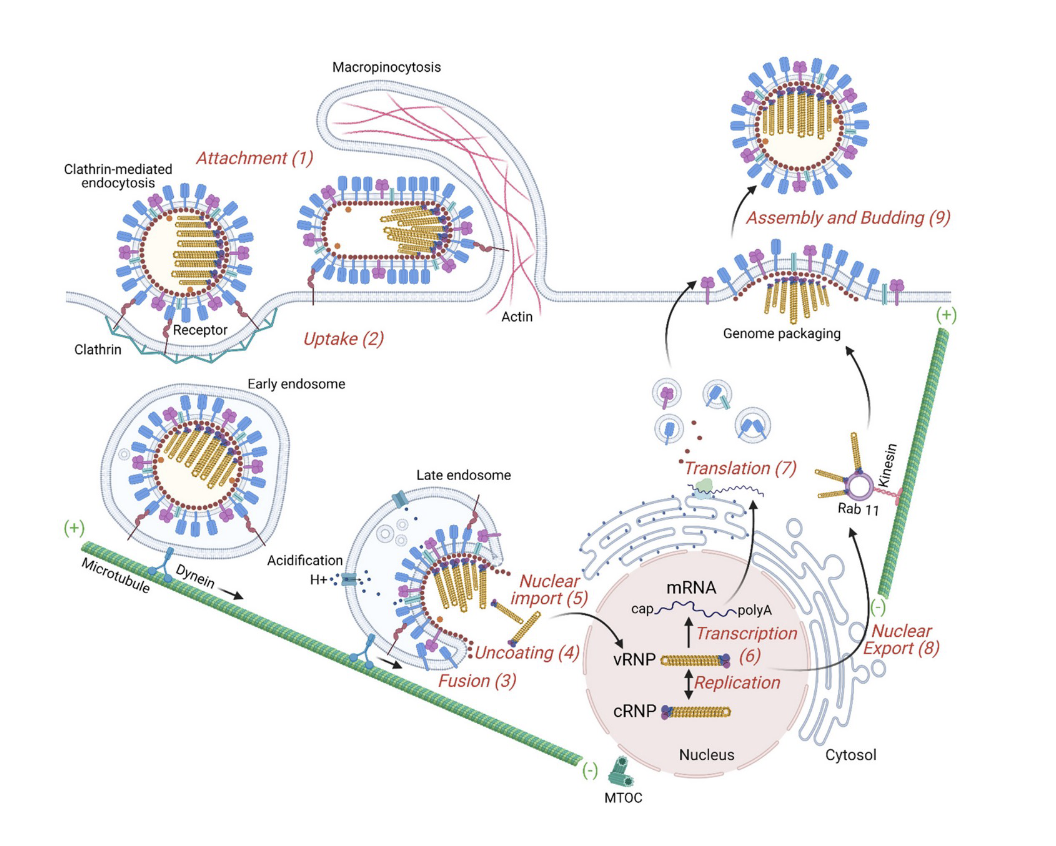
The HA protein (hemagglutinin) is shown in blue on the picture. So sialic acids on the surface of cells act as __ for the influenza virus.
what role does the HA proteins play with sialic acid base receptors?
receptors.
HA proteins bind to the sialic acid base receptors which then triggers the endocytosis of the influenza virus into the cell.
influenza and endocytosis/endosome:
once endocytosis occurs through early endosome which is this lipid-enclosed vesicle inside the cell.
normally the purpose of this process is that when something binds to the surface of the cell, we will take the material up and these early endosomes will mature into late endosome. these late endosomes start to decrease the pH of that compartment. the decrease of the pH of that compartment (this activates a bunch of proteases that destroy whatever it is being taken up into the cell. basically it is a way to grab nutrients from outside the cell) and fusion with lysosome.
the drop in pH in late endosome causes a conformational change in HA protein. it changes from binding to sialic acids to then the fusion peptide within the HA protein opens up and digs into the endosome. once the endosome is open, it releases the RNA nuclear particles associated with the influenza virus.
those RNA molecules are translated into protein. the new virus is produced and then the virus makes its way to the surface of the cell and escapes (the escape is called Budding). basically it buds into a new viral particle that can then go on to infect other cells.
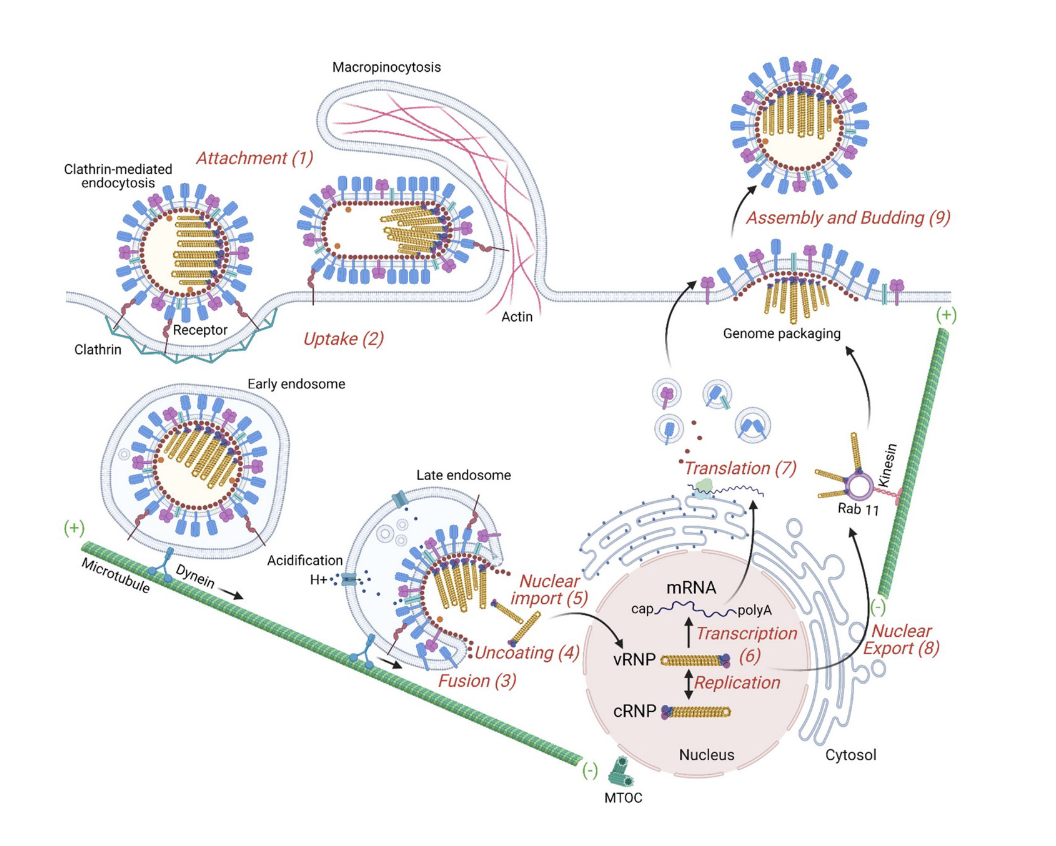
when this new viral particle buds, the other influenza surface protein called __ starts to play a really important role!
neuraminadase
Hemagglutinin protein (HA) is a dimer/trimer.
trimer.
neuraminadase protein is responsible for?
is a four polypeptide protein.
cleaving sialic acid.
it moves side to side and these lobes [where the active site of the enzyme are] can clip sialic acid residues off of proteins that are associated with receptors.
![<p>is a four polypeptide protein. </p><p>cleaving sialic acid.</p><p>it moves side to side and these lobes [where the active site of the enzyme are] can clip sialic acid residues off of proteins that are associated with receptors. </p>](https://knowt-user-attachments.s3.amazonaws.com/c7f23758-3508-4884-9b4c-0dc576fac95e.png)
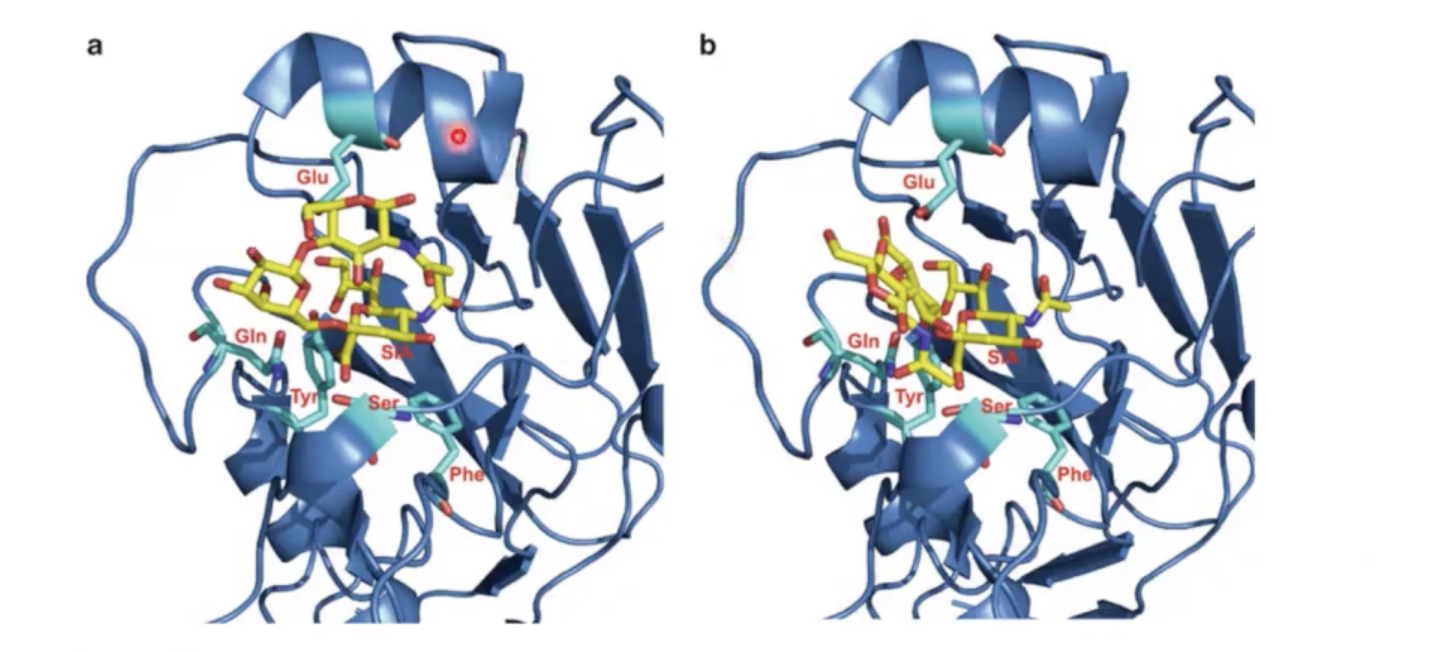
significance of this picture:
both dark blue proteins are hemagglutinin protein from birds (avian influenza virus).
however the sugar structures are different. In structure A, that sugar structure would mimic a human [α2,6-linked galactose]. the 2 is related to sialic acid and 6 is referring to the position of galactose that has been modified.
In structure B, that sugar structure would mimic a bird [α2,3-linked galactose]. notice that the sugars in binding site is packed better in structure B than structure A. This explains why bird influenza are difficult to infect humans.
what has to happen for it to be easier for bird influenza in human to transfer to another human easily?
these sugar (yellow) arrangements have to evolve to be able to accept α2,6- and α2,3 types of sialic acid surface modifications.
![<p>what does green and yellow show?</p><p>[this is a view of the neuraminadase protein]</p>](https://knowt-user-attachments.s3.amazonaws.com/1b77c281-0c97-416f-89d2-90ec55e27c02.png)
what does green and yellow show?
[this is a view of the neuraminadase protein]
green: catalytic site (the site where sialic acid normally binds. and you can cleave sialic acid from receptors.
yellow: secondary sialic binding site that is not in all strains of influenza virus. people are scared that this region is starting to adapt to different sialic acid structure where it might be able to impact another organism.
Tamiflu
neuraminadase inhibitor.
it inhibits neuraminadase so that the virus cannot escape.
The key to a functioning infective influenza virus is the right balance between __.
If there is too much HA relative to NA:
If there is too much NA vs HA:
HA and NA proteins on the surface.
the viral particle is not readily released to spread to other cells.
then the virus can never make it into the cell to begin with because the sialic acids were cleaved.