ACC (copy)
1/27
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
A typical Zisman plot is shown in the enclosed Figure. Explain the experiments that need to be made in order to obtain a Zisman plot.
To obtain a Zisman plot, a series of experiments involving measuring the contact angle of a liquid droplet on a solid surface with varying surface tension need to be conducted. This involves altering the surface tension of the liquid, plotting contact angles against the surface tension. This helps us figure out the critical surface tension needed for full wetting
What is meant by critical surface tension γc of a solid surface? What does this mean in reality?
the minimum surface tension required for a liquid to completely wet the surface.
Explain the difference between top-down and bottom-up approaches to prepare colloidal particles.
Top-down approaches involve breaking down larger particles into colloidal size, while bottom-up approaches involve building particles from smaller components.
Give two examples with short description on processes used in the top-down approach.
Examples of top-down processes include milling, where larger particles are mechanically reduced to colloidal size, and laser ablation, where intense laser beams break down materials into nanoparticles.
Give two examples with short description on different bottom-up approaches.
Examples of bottom-up processes include precipitation, where smaller particles nucleate and grow to form colloids, and condensation, where gas-phase particles aggregate to form colloidal structures.
Explain the main principle of steric stabilization of a colloidal system. What criteria need to be fulfilled to achieve good steric stabilization. What is meant by depletion stabilization?
Steric stabilization involves using polymers or surfactants to create a protective layer around colloidal particles, preventing aggregation. The criteria for good steric stabilization include the proper length of polymers to ensure effective coverage and prevent particle interactions. Depletion stabilization, on the other hand, occurs due to the presence of large solute molecules that create an effective repulsive force between colloidal particles, preventing their close approach and subsequent aggregation.
Polydispersity index:
Measures the distribution of particle sizes in a sample, providing insight into the uniformity of particle sizes within a colloid.
Symmetric unimodal distribution:
Particles are of similar size and form a single peak in the size distribution, indicating a uniform system.
Agglomerate and aggregate:
Agglomerates are loosely bound clusters, while aggregates are strongly bound clusters of particles, both influencing colloidal stability.
Physisorption vs chemisorption:
Physisorption involves weak physical interactions between molecules, while chemisorption involves chemical bonding, influencing the stability and reactivity of colloidal systems.
Charge reversal:
The change in the surface charge of colloidal particles under certain conditions, impacting their interactions and stability.
Peptisation:
The process of breaking down aggregates into colloidal particles, crucial for maintaining colloidal stability.
Rheopexy:
Thixotropic behavior, where a substance becomes temporarily gel-like under stress, affecting its flow properties.
Volume diameter and Surface diameter of colloidal particles:
Volume diameter represents the average diameter based on volume distribution, while surface diameter represents the average diameter based on surface area distribution of colloidal particles.
Resolution of Optical microscopy vs. TEM vs. SEM:
Technique | Resolution | What Can Be Seen | How It Works |
|---|---|---|---|
Optical Microscopy | ~200 nm | Larger particles, general structures | Visible light passes through the sample |
Transmission Electron Microscopy (TEM) | <0.1 nm (angstrom-scale) | Internal structures, nanoscale details | Electrons transmitted through the sample |
Scanning Electron Microscopy (SEM) | 1-10 nm | 3D surface imaging, surface morphology | Electrons reflected off the sample surface |
Small-angle X-ray scattering (SAXS) is a useful technique to obtain structural information about colloidal systems.Explain what information you can obtain from the Guinier, Porod and Bragg regions in the Figure.
Guinier Particle size
Porod structure of the aggregate
Bragg Interatomic distances
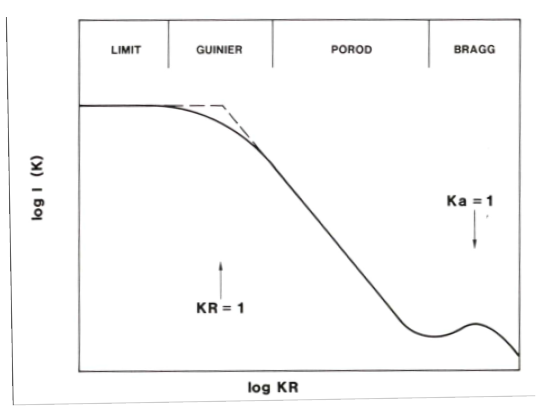
How can the fractal dimension of the system be determined from this plot? What does it tell?
Can be determined from the slope of the Guinier region. It measures the particle structure's complexity, with a higher fractal dimension indicating a more complex, less densely packed structure.
Name and shortly describe another scattering technique which you can use to obtain similar information on a larger length scale. what does it tell about the sample
Dynamic Light Scattering (DLS). DLS measures the fluctuations in scattered light intensity caused by Brownian motion of particles, providing information on particle size and size distribution.
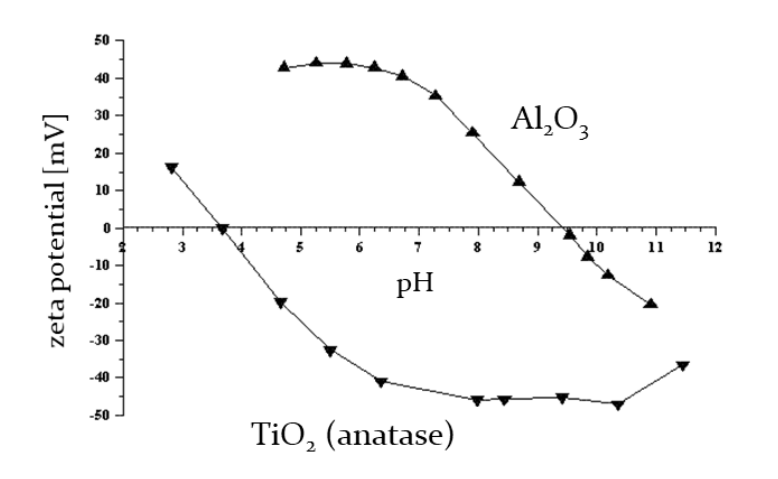
The enclosed Figure was obtained when measuring the zeta potential as a function of pH for aqueous dispersions of TiO2 and Al2O3 nanoparticles.Under which pH conditions can these dispersion be considered stable (well dispersed)? Why?
Both dispersions are considered stable at pH values where the zeta potential is far from zero (either strongly positive or negative). This indicates a repulsive force preventing particle aggregation. For example, at pH 3 for TiO2 and around pH 6.75 for Al2O3, the dispersions are likely to be stable.
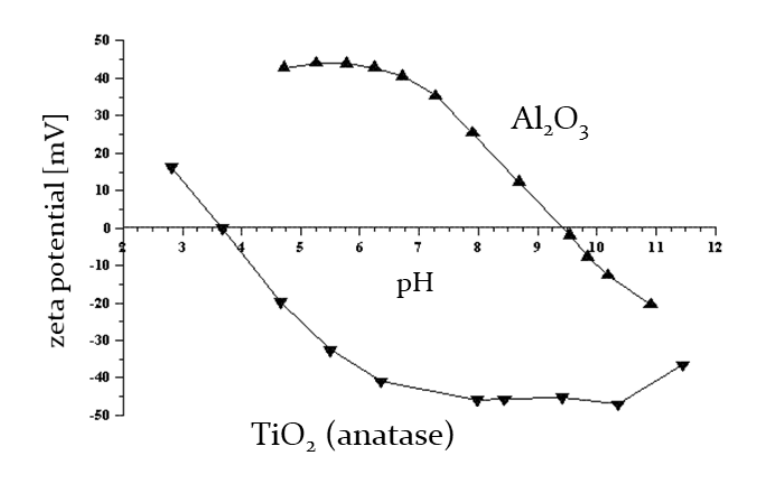
What is the isoelectric point for these systems?
The isoelectric point is the pH at which the zeta potential is zero. For TiO2, it's around 3.5, and for Al2O3, it's around 9.5. At the isoelectric point, the repulsive forces are minimal, leading to increased chances of particle aggregation.
Shortly describe at least two different methods for measuring the zeta potential.
Electrophoretic light scattering (ELS) measures the electrophoretic mobility of charged particles in an electric field,
Laser Doppler velocimetry (LDV) uses the Doppler shift in laser light scattered by particles to determine their velocity, providing information on zeta potential.
Phase Analysis Light Scattering (PALS) analyzes the phase shift of scattered light due to the Brownian motion of particles.
As discussed in the lecture, sedimentation of spherical particles follows Stoke's law: f = 6 π η a, where η = the viscosity of the medium and a = the radius of the particles. For asymmetric and/or solvated particles one can define a frictional ratio (f/f0), which is the actual frictional coefficient, f, compared to that of an unsolvated sphere, f0. Based on the enclosed Figure (protein particles), answer the following questions: In which way is the frictional coefficient changing for (i) asymmetric particles (with axial ratio a/b) and (ii) solvated particles?
a) For Asymmetric Particles: As the axial ratio a/b increases, the frictional coefficient also increases, indicating that elongated or asymmetric particles experience higher resistance to motion in the medium, leading to greater frictional forces.
b) For Solvated Particles: As the hydration mass of water/mass of protein increases (indicating higher solvation), the frictional coefficient also increases. This increase in water content makes the particles more adhesive in the solution. This suggests that solvated particles experience an elevated level of friction compared to unsolvated particles.
In rheology, how is this chart related to the viscosity of the sample?
The viscosity of the sample is related to the frictional coefficient. As the frictional coefficient increases, the viscosity of the sample also increases. This relationship is crucial in understanding the flow behavior of colloidal suspensions in different conditions.
The DLVO theory can be used to predict the stability of a colloidal suspension. The total potential energy is the sum of the repulsive, attractive and steric contributions: ΦT = ΦR + ΦA + ΦS. In which ways can the magnitude of each of these three contributions be affected?
Repulsive (ΦR): Affected by surface charge, electrolyte concentration, and particle size. Increased charge or electrolyte concentration increases repulsion. Attractive (ΦA): Influenced by van der Waals forces. Higher particle-particle distances result in weaker attractive forces. Steric (ΦS): Determined by polymer or surfactant coverage. Increased coverage enhances steric repulsion.
Under certain conditions, especially for large particles, a colloidal system can display both a primary and a secondary minimum (as shown in the picture). What fundamental differences do these minima represent?
The primary minimum represents a stable equilibrium point where attractive van der Waals forces dominate, favoring particle aggregation. The secondary minimum is a less stable position where particles can temporarily linger due to repulsive forces counteracting attractive forces.
Nucleation and growth:
Nucleation is the initial step in particle synthesis where small clusters of atoms or molecules form and serve as the building blocks for particle growth. During growth, these clusters accumulate material, leading to the formation of larger particles. The control of nucleation and growth parameters is crucial for achieving desired particle size, uniformity, and properties in various applications, from drug delivery to materials science.
Spray drying
is a technique where a liquid solution or suspension is atomized into fine droplets and then dried to produce solid particles. It is widely used for the production of powders with controlled particle sizes and morphologies
Explain what the adsorption isotherm (IV) below tells about the porosity of the analyzed sample.
The type IV adsorption isotherm suggests that the analyzed sample exhibits a mesoporous structure. In the initial stages, as relative pressure increases, there is a slow and gradual adsorption, indicating the filling of mesopores. The rise in volume adsorbed at higher relative pressures is indicative of the condensation of gas in larger mesopores and the onset of capillary condensation. The hysteresis loop, typically associated with type IV isotherms, signifies the desorption of gas from the mesopores, emphasizing the mesoporous nature of the material. The shape and characteristics of the type IV isotherm reflect a material with well-defined mesoporous properties, providing valuable insights into its porosity.