Enthalpy + Entropy
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Enthalpy is what?
Heat AKA the change in energy if a system
At constant pressure: Change in enthalpy =
heat flow
Enthalpy is what type of function?
A state function
In a state function
path is independent
Enthalpy is positive (+) in what reactions?
Endothermic Reactions
Enthalpy is negative (-) in what reactions?
Exothermic Reactions
Endothermic Reactions:
Heat enters system (reactants + heat → products)
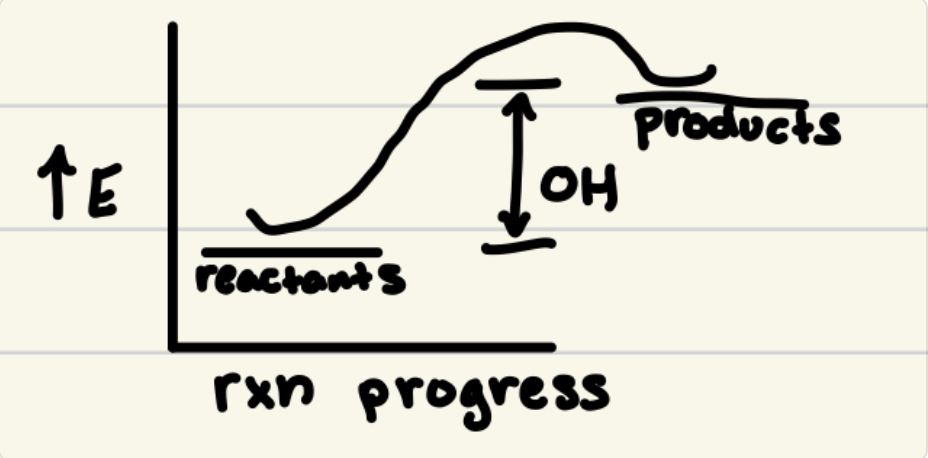
Exothermic Reactions:
Heat leaves system (reactants → products + heat)
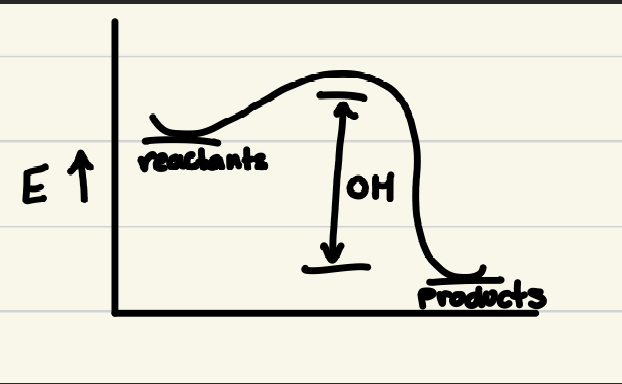
Equation for Enthalpy:
H = U + PV
H:
U:
P:
V:
Enthalpy
Internal Energy
Pressure
Volume
Standard enthalpy change reaction occurs at:
25 C and 1 atm
Standard enthalpy of formation (DeltaH*f) is the change in enthalpy for the reaction that forms…
one mole of the compound from it’s elements in their standard states
Standard enthalpy of formation is 0 for:
Elements by itself including diatomic and when at standard state
If you flip a reaction should you change the sign of that ΔH?
Yes
If you multiply a reaction should you also multiply the ΔH?
Yes
Does enthalpy make a reaction spontaneous?
No
Combustion reaction involves what?
Its a fast, exothermic reaction involving a fuel and oxygen
Entropy measures what?
The randomness/disorder of a system
Entropy is a state function T/F?
True
Entropy is temperature dependent T/F?
True
How to find ΔSuniverse
ΔSuniverse=ΔSsystem+ΔSsurroundings
At Standard state, S is what?
1 bar for gases, liquids and solids
1 M for solutions
S _________ (slightly) as temperature rises
increases
S____________ as physical state becomes less ordered (as you go from solid to liquid to gas)
(solid < liquid<<gas)
increase
S often (but not always) ________ when substances are mixed. (An important exception is when gas is dissolved in a liquid)
increase
S__________as the number of molecules increases
increase
S usually___________ as molecules or atoms get bigger and more complicated
increase (size bigger = more room for chaos)
In a favorable/spontaneous reaction, the sign of ΔS is what?
Positive (+)
In a favorable/spontaneous reaction, the sign of ΔH is what?
Positive (+)
If you go from 3 moles of gas to 2 moles of gas, what does this mean?
less molecule = less chaos = Entropy (ΔS) decrease
Heat and entropy are what?
Proportional
Heat transferred to the surroundings raises entropy of the what?
surroundings
Temperature has small impact on entropy, but big impact on what?
spontaneous or not
If ΔG < 0 then ΔS is
> 0 spontaneous (exergonic)
If ΔG > 0 then ΔS is
< 0 Non-spontaneous (endergonic)
If ΔG = 0 then ΔS is
= 0 Equilibrium
What equation do you use to find free energy?
ΔG = ΔH - TΔSsys
ΔG is a ___________ function
state