chemical analysis
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
How do you test for hydrogen gas?
Place a lit splint at the mouth of a test tube containing the gas.
What is the positive result for hydrogen?
A squeaky pop sound is heard.
Why does hydrogen make a squeaky pop?
Hydrogen reacts explosively with oxygen in the air to form water (H₂O).
How do you test for oxygen gas?
Insert a glowing splint into a test tube containing the gas.
What is the positive result for oxygen?
The glowing splint relights.
Why does oxygen relight a glowing splint?
Oxygen supports combustion, allowing the splint to reignite.
How do you test for carbon dioxide gas?
Bubble the gas through limewater (aqueous calcium hydroxide).
What is the positive result for carbon dioxide?
The limewater turns cloudy (milky white).
Why does limewater turn cloudy in the presence of carbon dioxide?
Carbon dioxide reacts with calcium hydroxide to form calcium carbonate (CaCO₃), which is insoluble and appears as a white precipitate.
What happens if excess CO₂ is bubbled through limewater?
The cloudy solution clears again because calcium carbonate reacts with more CO₂ to form soluble calcium hydrogen carbonate.
How do you test for chlorine gas?
Hold a piece of damp blue litmus paper in the gas.
What is the positive result for chlorine?
The blue litmus paper turns red and then white.
Why does chlorine turn blue litmus paper red first?
Chlorine is acidic in water, so it temporarily makes the litmus paper red.
Why does chlorine then turn the litmus paper white?
Chlorine is a bleaching agent and removes the colour from the paper.
How do you test for halide ions (chloride, bromide, iodide)?
Add dilute nitric acid (HNO₃) followed by silver nitrate (AgNO₃) solution.
What are the results for different halide ions?
Chloride (Cl⁻) → White precipitate (AgCl)
Bromide (Br⁻) → Cream precipitate (AgBr)
Iodide (I⁻) → Yellow precipitate (AgI)
Why is nitric acid added before silver nitrate?
To remove carbonate impurities, which could also form a precipitate.
what is the general ionic equation for the formation of the precipitate from halide ion
Ag+ + X- ——> AgX(s)
how to test for a carbonate
add dilute acid
bubble the gas through limewater
if CO2 is present, effervescence then CO2 gas is formed turning the limewater cloudy
- make sure you connect the test tube of the suspected ion to the test tube of limewater quickly so that none of the CO2 escapes
How do you carry out a flame test?
Dip a clean nichrome wire loop into a sample of the solid, then place it in a blue Bunsen burner flame and observe the colour.
why must a blue bunsen burner flame be used
because a yellow flame isn’t hot enough and it may mask the colours being made by some ions
Why must the wire loop be cleaned before each test?
To remove contamination that could affect the flame colour by masking colours produced by ions or by two or more ions being present on the wire and so the colours mix
how to clean the wire before each flame test
place it in acid first to avoid contamination
lithium colour change flame test
Lithium (Li⁺) → Crimson red
sodium colour change flame test
Sodium (Na⁺) → Yellow
potassium colour change flame test
Potassium (K⁺) → Lilac
calcium colour change flame test
Calcium (Ca²⁺) → Orange-red
copper colour change flame test
Copper (Cu²⁺) → Green
How do you test for metal cations using sodium hydroxide?
Add sodium hydroxide (NaOH) solution to the sample and observe the colour of any precipitate formed.
Ca NaOH test results
Calcium (Ca²⁺) → White precipitate
Mg NaOH test results
Magnesium (Mg²⁺) → White precipitate
Al NaOH test results
Aluminium (Al³⁺) → White precipitate (dissolves in excess NaOH)
Cu NaOH test results
Copper(II) (Cu²⁺) → Blue precipitate
Fe2+ NaOH test results
Iron(II) (Fe²⁺) → Green precipitate3+
Fe3+ NaOH test results
Iron(III) (Fe³⁺) → Brown precipitate
How can you distinguish between calcium and magnesium ions in the NaOH test?
Use a flame test – calcium gives an orange-red flame, while magnesium has no flame colour.
How do you test for sulfate ions?
Add dilute hydrochloric acid (HCl) followed by barium chloride (BaCl₂) solution.
What is the positive result for sulfates?
A white precipitate of barium sulfate (BaSO₄) forms.
Why is hydrochloric acid added before barium chloride?
To remove carbonate impurities, which could also form a white precipitate.
What is flame emission spectroscopy?
It is an instrumental method used to identify metal ions and determine their concentration by analysing the light they emit when heated.
What type of elements does flame emission spectroscopy detect?
Metal ions, particularly transition metals.
how to identify which metal ions are present in an unknown sample
look for which metal ions have the same lines as the unknown sample does
Why is flame emission spectroscopy useful?
very accurate
very sensitive
very fast
How is the light analysed?
The light passes through a spectroscope, which separates it into a unique line spectrum of different wavelengths.
Why does each metal ion produce a unique line spectrum?
Because each element has a unique electron arrangement, so it emits specific wavelengths of light so leading to different observed colours
How can flame emission spectroscopy be used to identify metal ions?
Each metal ion has a unique pattern of spectral lines, which can be matched to known reference spectra.
How can flame emission spectroscopy determine the concentration of a metal ion?
The intensity (brightness) of the spectral lines is proportional to the concentration of the metal ion in the sample.
What are the advantages of flame emission spectroscopy over flame tests?
More sensitive – can detect very small amounts of a substance.
More accurate – avoids errors from subjective colour observations.
Can analyse mixtures – unlike simple flame tests, which only show one dominant colour.
Very fast - can usually be automated
Where is flame emission spectroscopy used?
Forensic science – identifying metals in crime scene samples.
Environmental monitoring – detecting metal pollution in water.
Industrial processes – ensuring correct metal compositions in alloys.
Health applications – measuring metal ions in blood samples.
What is a pure substance in chemistry?
A pure substance contains only one type of element or compound with no other substances mixed in.
How can you test the purity of a substance?
By measuring its melting or boiling point and comparing it to the known values of the pure substance.
How does the presence of impurities affect the melting and boiling points?
Impurities lower the melting point and increase the boiling point, often causing a range of temperatures rather than a sharp change.
What is a formulation?
A formulation is a mixture designed to produce a useful product with specific properties, made by mixing different substances in precise amounts.
Give three examples of formulations.
Paints, fuels, medicines, alloys, fertilisers, and cleaning agents.
Why are formulations important in the pharmaceutical industry?
The precise combination of active and inactive ingredients ensures the medicine is effective, safe, and delivers the correct dose.
What is chromatography used for?
To separate mixtures and identify substances based on their movement through a stationary phase.
What are the two phases in chromatography?
The mobile phase (solvent) and the stationary phase (paper or solid material).
How does chromatography work?
Substances move with the mobile phase at different rates due to differences in solubility and attraction to the stationary phase.
What is an Rf value?
The retention factor (Rf) is a ratio that measures how far a substance moves in chromatography relative to the solvent front.
How do you calculate the Rf value?
Rf=distance moved by substance/ distance moved by solvent
Why do different substances have different Rf values?
Because they have different solubilities in the solvent and attractions to the stationary phase.
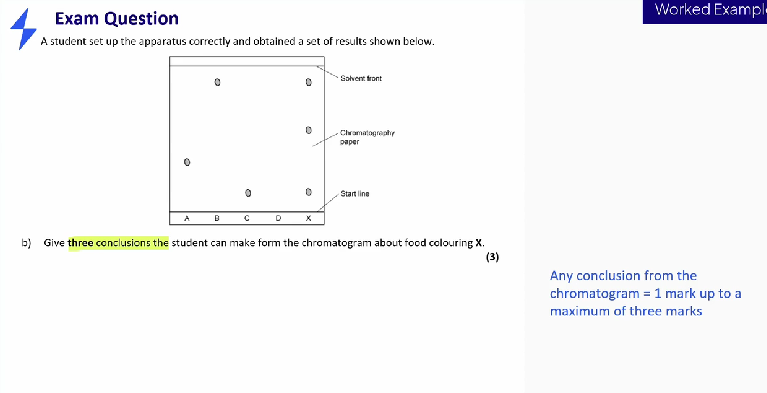
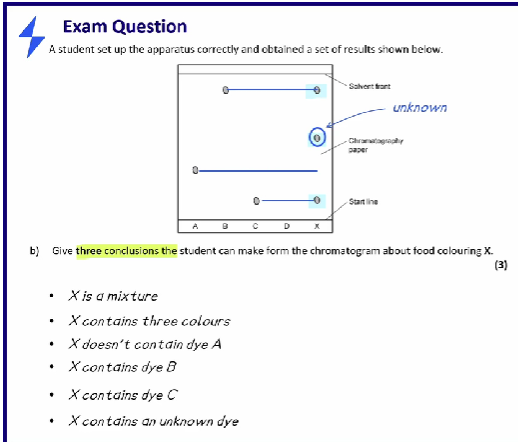
Give conclusions that can be made from this chromatogram
- X is a mixture
- X contains three colours
- X doesn’t contain dye A
- X contains dye B
- X contains dye C
Why must the starting line in paper chromatography be drawn in pencil?
Pencil does not dissolve in the solvent, unlike ink, which could interfere with the results.
describe the stationary phase
substance the molecules can’t move in so usually a solid. In this case it’s the paper so the substances bind to the paper. Substances less soluble are more attracted to the paper and so spend more time in the stationary phase so move up the paper slower
how to carry out chromatography
get a beaker, fill it with a shallow amount of solvent.
draw a pencil baseline on chromatography paper
place paper in beaker and mak sure the baseline remains above the solvent and they don’t submerge
place a lid on top so that the solvent doesn’t evaporate
wait for the solvent to seep up the paper and mark the solvent front
dry the chromatography paper
measure distance travelled by solvent so distance between start line and solvent front
measure distance between start line and spot to calculate distance travelled by the substance
describe the mobile phase
substances the molecule can move in eg. liquid or a gas, in this case it’s the solvent we use, the chemicals more soluble in the solvent spend more time in the mobile phase and so move up the paper faster