Unit 3 - Composition of Substances and Solutions
1/19
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Aqueous Solution
A solution where water is the solvent example) Saltwater (NaCl dissolved in water)
Avogadro’s Number (NA)
Number of particles in 1 mole of a substance, equal to 6.022 × 10²³
example) 1 mole of oxygen molecules has 6.022 × 10²³ O₂ molecules
Concentrated
A solution with a relatively large amount of solute
example) Strong coffee has a concentrated amount of caffeine
Concentration
The measured amount of solute compared to solvent in a solution
example) 1 M NaCl solution has 1 mole of NaCl in 1 L of water
Dilute
A solution with a relatively small amount of solute
example) Weak lemonade with little sugar
Dilution
Process of adding more solvent to lower solute concentration
example) Adding water to orange juice makes it less concentrated
Dissolved
When a solute spreads out evenly in a solvent
example) Sugar dissolving in tea
Empirical Formula Mass
The total average mass of atoms in the simplest formula of a compound
example) CH₂O (glucose’s empirical formula) has a mass of about 30 g/mol
Formula Mass
Sum of the average atomic masses of all atoms in a chemical formula
example) H₂O has a formula mass of about 18 amu
Mass Percentage
The mass of solute divided by total solution mass × 100
example)
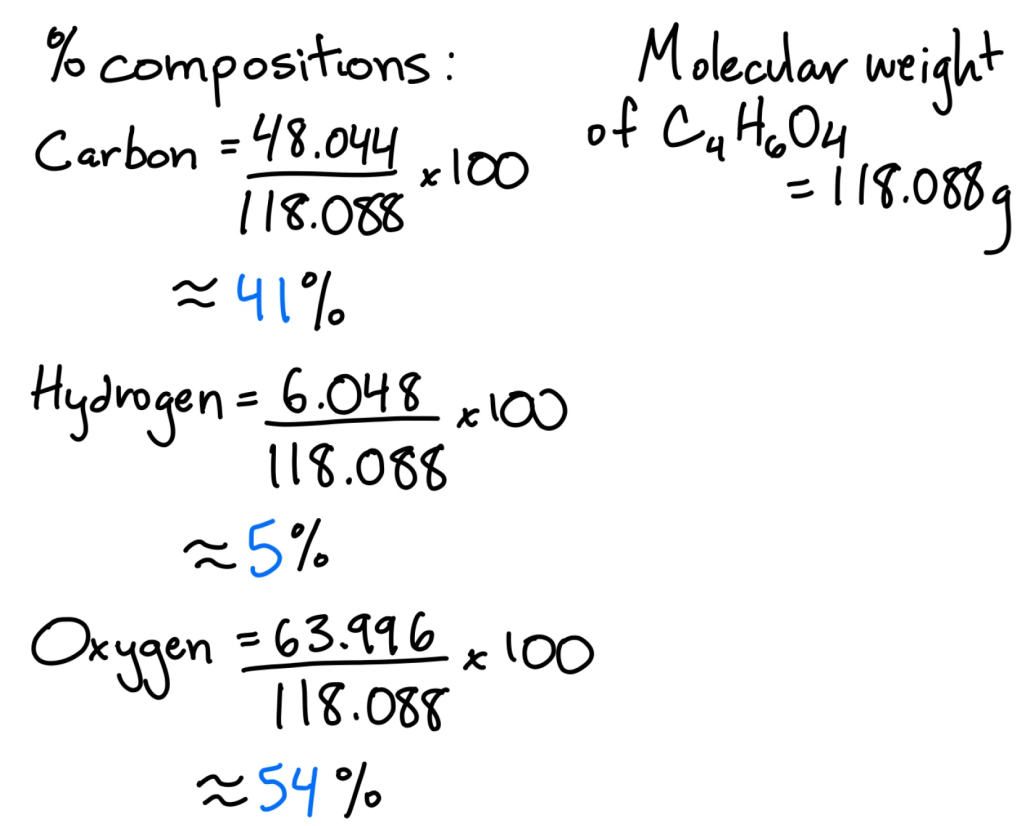
Mass-Volume Percent
Solute mass divided by solution volume × 100
example) 10 g sugar in 100 mL solution = 10% (m/v)
Molar Mass
The mass in grams of 1 mole of a substance
example) 1 mole of H₂O = 18 g
Molarity (M)
Concentration unit, moles of solute per liter of solution
example) 0.5 M NaOH has 0.5 moles of NaOH in 1 L of solution
Mole
Amount of substance with 6.022 × 10²³ particles
example) 1 mole of carbon atoms = 6.022 × 10²³ atoms
Parts per Billion (ppb)
Solute-to-solution mass ratio × 10⁹
example) 1 µg solute in 1 L solution = 1 ppb
Parts per Million (ppm)
Solute-to-solution mass ratio × 10⁶
example) 1 mg solute in 1 L solution = 1 ppm
Percent Composition
Percent by mass of each element in a compound
example) H₂O is 11.2% H and 88.8% O
Solute
The part of a solution present in the smaller amount
example) Salt in saltwater
Solvent
The part of a solution present in the larger amount
example) Water in saltwater
Volume Percentage
Solute volume divided by solution volume × 100
example) 40 mL ethanol in 100 mL solution = 40% (v/v)