Chemistry - Test 2 (Atomic Structure/Nuclear Chemistry)
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Atom
Smallest particle of an element that retains properties of the element
Protons
Positively charged subatomic particle; found in nucleus of atom
Electrons (e–)
Negatively charged subatomic particle; found around nucleus
Neutron
No charge subatomic particle; found in nucleus
Nucleus
Small center of the atom
Atomic number
Same as the number of protons
Mass number
Total number of protons and neutrons in an atom
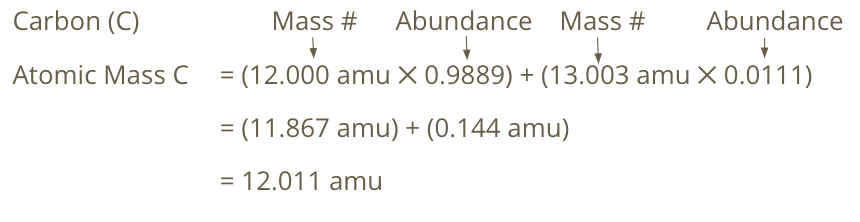
Atomic Mass
Average mass of all naturally occurring isotopes of an element
Isotope Abundance
Amount a given isotope is found in nature, usually measured as a percentage
Isotope Notation
A way of abbreviating the name of an isotope
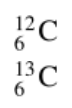
Radioactivity
Spontaneous emission of rays or particles from elements
Alpha radiation
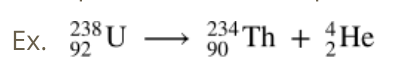
Beta Radiation
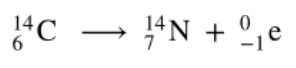
Alpha decay
When helium (He) nuclei is emitted as an alpha particle
Beta decay
A neutron breaks apart into a proton and electron
Gamma Rays
High energy rays emitted by radioactive isotope
Gamma decay

Electron Capture
Occurs when nuclei have too few neutrons; Converting of a proton to a neutron, e– is captured
Electron capture equation
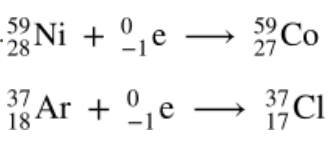
Positron Emission
Proton changes to a neutron
Positron (e+)
Particle with mass of e– but a positive charge; form of anti-matter
Positron Emission equation
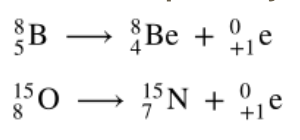
Alpha particle
Helium-4
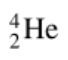
Beta Particle
An electron or a positron
Half Life
Amount of time for half of the nuclei to decay
Calculate Half-life
A = A0 X (1/2)n
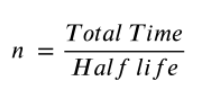
Nuclear Fission
The Process of bombarding certain isotopes with neutrons, causing them so split
Nuclear Fusion
Occurs when nuclei combine to from a heavier nucleus; usually Hydrogen (H)
Radioisotope
An unstable form of a chemical element that releases radiation as it breaks down and becomes more stable
Transmutation
Converting of an element to a different element; Can occur through decay or particles bombarding nucleus
Transuranium elements
Elements with atomic number >92; These elements are synthesized with nuclear accelerator
Calculating Atomic Mass
Multiply the mass # by the abundance of each isotope in nature, then add up the different isotopes
Calculating Mass Number
Protons + Neutrons
Density Calculation
Mass/Volume