Quantum phenomena and emission spectra
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is excitation?
When an electron absorbs enough energy to move up to a higher energy level. To excite an electron, it must absorb a photon, to de-excite it must emit a photon.
Which energy level do atoms naturally occupy?
The lowest energy level possible as it is the most stable.
What is ionisation?
When an atom gains or loses an orbital electron and becomes charged. Electrons can be removed from any energy level of an atom.
What is the ionisation energy of an atom?
the minimum energy required to remove an electron from the ground state of an atom
Explain how a fluorescent tube emits light.
Voltage is applied across the tube, electrons flow from the cathode to the anode producing an electron beam
Beam electrons collide with the electrons in the mercury atoms, transferring kinetic energy in the collision
The atomic electrons in the mercury atoms are excited and move to a higher energy level
This high energy level state is unstable and so the electrons de-excite, move back to their ground state
As they de-excite, the electrons release energy by emitting photons in the UV range of wavelengths
The UV photons then collide with electrons in the atoms of the phosphor coating and excite them into a higher energy level
As these phosphor electrons de-excite, they do so in stages emitting photons in the visible light range of wavelengths
Tubes also contain argon gas to prevent tungsten filament from corroding/oxidising
What is the ground state?
The lowest state of energy for a specific electron
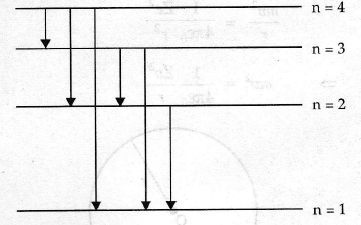
Label this diagram
n=1 is the ground state. n=4 is the ionisation level. Other levels are excitation levels. Energies in between correspond to energy needed to excite an electron to the next energy level/energy of emitted photon as electrons de-excite.
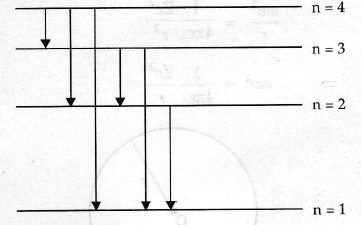
What are line spectra/When do they occur?
a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours
each element produces a unique set of line spectra (they can be identified by this)
Two types: emission and absorption spectra
They are evidence for the discrete energy levels in atoms
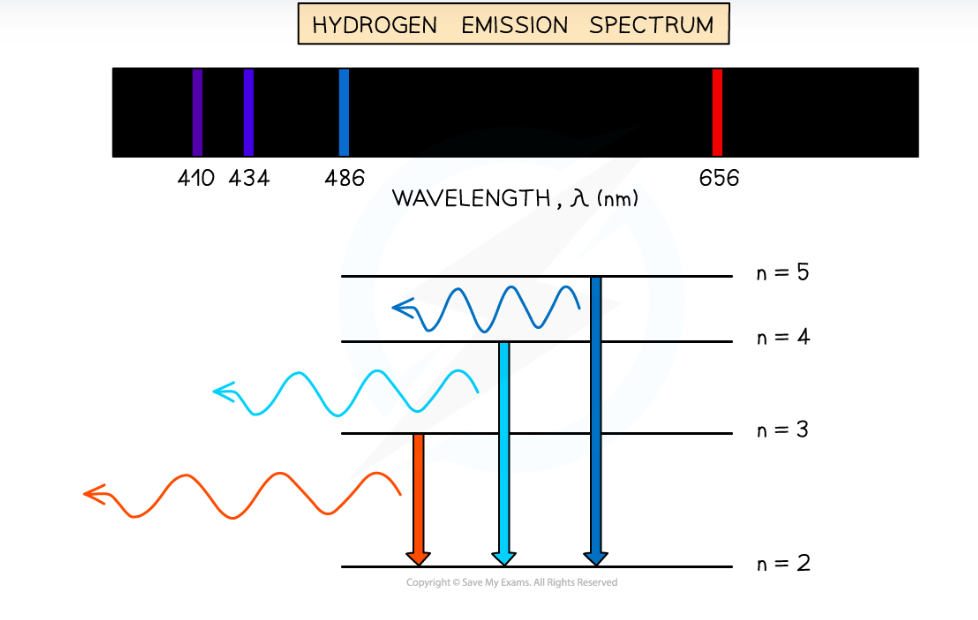
What are emission spectra?
Line spectra that are recorded when an electron transitions from a higher to lower energy level, emitting a photon.
Each transition corresponds to a wavelength of light
Characterised by a set of discrete wavelengths represented by coloured lines on a black background
What do line spectra evidence?
they provide evidence that electrons only travel between discrete energy levels.
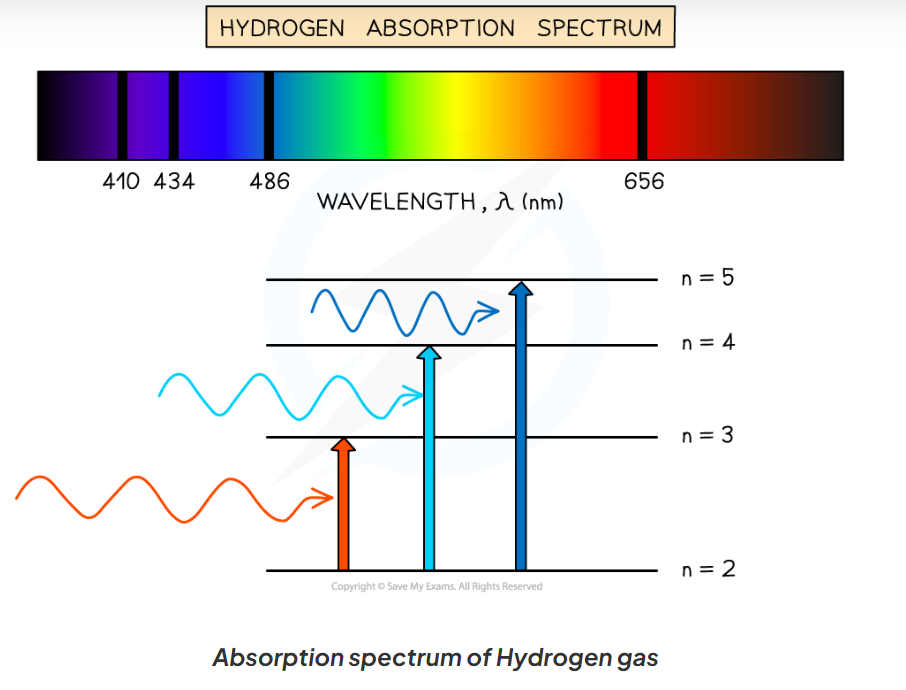
What are absorption spectra?
When white light is passed through a cool low pressure gas, photons with specific wavelengths that are being absorbed by the gas to excite electrons. These are not transmitted to the spectrum and any photons emitted are emitted in all directions. This causes light of specific wavelengths to be missing from the spectrum.
the rest of the light is transmitted through the gas
they match up exactly with emission spectra for the same element.
How are absorption spectra characterised?
A continuous spectrum with dark lines at specific wavelengths
dark lines correspond to energy levels in an atom, and emission spectra
Equation for the difference between two energy levels
ΔE = hf = E2 - E1
Where E1 is the energy of the higher level and E2 is the energy of the lower level.
How does the electron diffraction pattern support the idea that the electron beam behaves as a wave?
Particle behaviour would only produce a patch/circle of light/small spot of light or particles would scatter randomly.
Diffraction and interference are wave properties
Graphite causes electron beam to spread out
Maximum intensity occurs where waves interfere constructively; they are in phase
How does the emission of light from the fluorescent screen show that the electrons behave as particles.
Electrons must provide enough instantaneous energy to cause excitation
Electron can provide energy in discrete amounts
Energy cannot be provided over time as it would be in a wave
Incident electrons collide with orbital electrons transferring kinetic energy. This discrete energy excites the orbital electrons, when they de-excite they emit photons.