antibiotic drug classes and mechanism
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
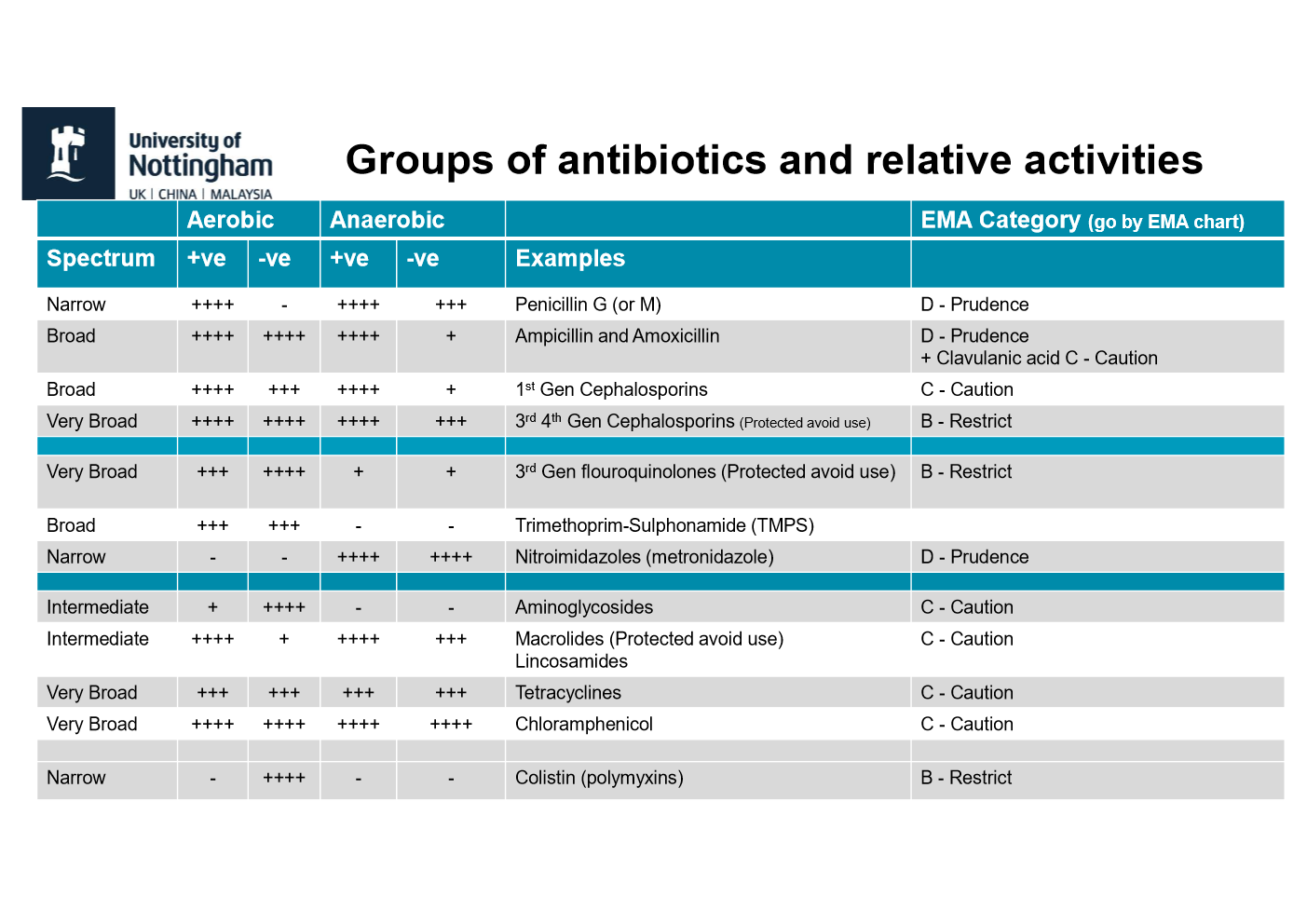
what are two spectrums of activity in antibiotics?
narrow and broad
what is the magic bullet
• The magic bullet is a term coined to reflect the optimum antibiotic.
why can ab have adverse reactions
Some antibiotic will however impact on the host.
membrane interacting antibiotics while having a higher affinity for bacterial membranes may interact a high concentrations with patient membranes so can have unintentional side effects when administered in certain ways.
what is narrow spectrum of antibiotics?
§Targets narrow group of bacteria (i.e. either Gram positive or Gram negative)
what is broad spectrum of antibiotics?
affect a broad range of gram-positive and gram-negative bacteria
what impact will broad spec ab have
more impact on the other bacteria in the host and cause more disruption.
wider impact on a range of bacteria is considered a bad idea in relationship to management and reduction of risk for resistance.
what are the two killing activities in bacteria?
bacteriocidal, bacteriostatic
bacteriocidal and examples
§Kills the organism
§Example penicillin's, cephalosporin's
bacteriostatic and examples
§Drugs that temporarily inhibit the growth of an organism (i.e. reversible if removed).
§Examples Tetracycline's, Chloramphenicol
What is the difference between bacteriocidal and bacteriostatic antibiotics?
Bacteriocidal antibiotics kill bacteria, while bacteriostatic antibiotics inhibit bacterial growth and rely on the host's immune system to eliminate the pathogens.
When are bacteriocidal antibiotics preferred?
Bacteriocidal antibiotics are preferred in patients who are immunocompromised or when there is limited immune control at the infection site. in critical care → host defences where they wont be able to manage the infection
What does the distribution of an antibiotic depend on?
Its chemistry.
What is necessary for an antibiotic to be effective at the site of infection?
It needs to reach the MIC (minimum inhibitory concentration).
What does MIC stand for?
Minimum inhibitory concentration.
What does MBC stand for?
Minimum bactericidal concentration.
Do the MIC and MBC values for antibiotics vary?
Yes, they vary by genera/species of bacteria.
what happens to the drug conc during course and when the bact ebcome resistant
course: conc is well above the MIC
resis: their MIC goes up
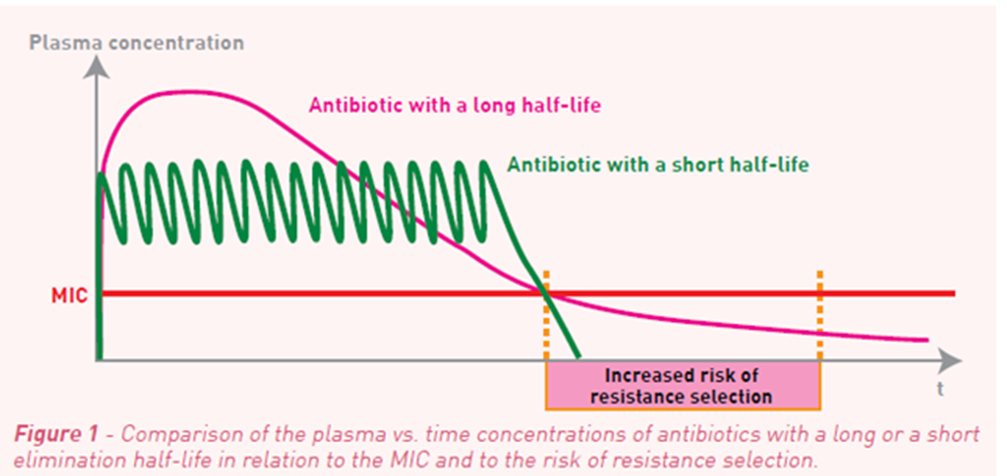
what does the concentration of an ab need to be to treat infection
To treat an infection the antibiotic needs to reach the concentration higher than the MIC of the bacteria cause the infection for long enough to stop (bacteria) or kill the bacteria and for the host to clear the infection.

What is concentration dependent killing?
Concentration dependent killing is where the drug has to be at or above the critical concentration to have an impact.
What does concentration dependent killing relate to?
It relates to getting enough of the drug bound to the targets in the bacteria to have an effect.
What is time dependent killing?
Time dependent killing refers to antibiotics that take time to exert their effect, requiring the dose to be maintained.
What happens if a bacteriostatic antibiotic is removed too quickly?
If a bacteriostatic antibiotic is removed too quickly, the bacteria will start growing again.
what are some targets of antibiotic action?
1. inhibition of protein synthesis
2. disruption of DNA structure
3. inhibition of membrnae function
4. inhibition of cell wall synthesis
5. interference with other pathways
6. inhibition of DNA dependant RNA polymerase
What is unique to bacteria that makes it a good target for antimicrobial treatment?
Peptidoglycan
What is peptidoglycan composed of?
A polymer of sugars and amino acids
What structure does peptidoglycan form in bacterial cells?
A mesh-like cell wall
What enzyme produced by the host breaks bonds in the sugar polysaccharide of peptidoglycan?
Lysozyme
What type of antibiotic inhibits the transpeptidase (PBP) to prevent peptide cross-links from forming?
Beta-lactam antibiotic
What do glycopeptides do to the peptide chains in peptidoglycan?
They cap the peptide chains to prevent cross-linking
What is the result of weakened cell walls in bacteria?
Rupture due to positive osmotic pressure
how do beta lactam antibiotics target the cell wall?
Penicillin binding protein (it is a transpeptidase). Inhibition stops peptide cross links in wall.
how do peptide antibiotics, (glycopeptides and vancomycin) target the cell wall?
Directly interacts with cell wall D-Ala-D-Ala moieties Preventing synthesis of the peptidoglycan cross linking.
how does bactiracin target the cell wall?
Cyclic peptides that interfere with dephosphorylation of isoprenyl carriers for cell wall synthesis.
how does teixobactin target the cell wall?
Binds to a highly conserved motif of lipid II (precursor of peptidoglycan) and lipid III (precursor of cell wall teichoic acid)
how are beta lactams split
Penicillin's and Cephalosporins.
They have the square beta lactam ring in common and both inhibit the penicillin binding protein (PBP) which is a transpeptidase making bonds in the cell wall.
• Some groups can not penetrate the outer member of the Gram-negative bacteria so have a narrow spectrum only working on Gram-positive bacteria.
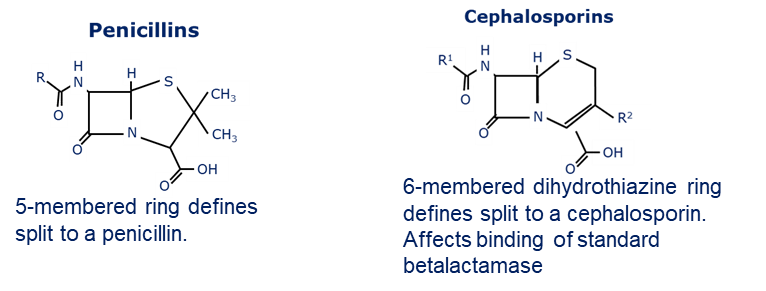
why is resistance to penicillin high?
due a beta-lactamase enzyme that breaks the lactam ring.
why is methicillin useful
• Methicillin is useful because it is tolerate of the beta-lactamase however there is a specific resistance due to a duplicate penicillin binding protein in bacteria that causes methicillin and other beta-lactam resistances (see your year two MRSP session for notes)
how do the different generations of the cephalosporins act
1st generation cephalosporins very effective against Gram-positive bacteria but are only moderately effective against Gram-negative bacteria.
2nd generation cephalosporins target both Gram-positive and Gram-negative bacteria. but are considered less effective against Gram-positive bacteria compared to 1st gen. Ineffective against MRSA
3rd and 4th generation boarder activity, but often considered reserved for critical use in human medicine.
5th Gen only one major group in use in US and for critical treatment where other options do no work in humans. Currently effective against MRSA.
how to antibiotics inhibit protein synthesis?
Protein-synthesis–inhibiting antibiotics bind to the bacterial ribosome and block different steps of translation.→ Without proteins, bacteria stop growing or die.
how are ribosome targeting antibiotics selective
They are selective (magic bullet) due to structural differences between the 80S (eukaryotic) and 70S (prokaryotic) ribosome
examples of ribosome binding antibiotics
bacteriocidal
aminoglycosides
nitrofurans
bacteriostatic
tetracyclines
chloramphenical
macrolides
lincosamides, and pleuromitilins
action of aminoglycosides
Bind the 30S and effect a number of steps in protein synthesis leading to non-functional proteins.
Causes irreversible inhibition
action of nitrofurans
It is reduced by bacterial flavoproteins to reactive intermediates that inhibit bacterial ribosomes and other macromolecules.
action of Tetracyclines
•Oxytetracycline
•Doxycyline action
Enter cell by active process and bind to receptors on the 30S subunit.
They block tRNA attachment.
describe the activity and pharmacology of aminoglycosides
•Predominantly Gram –ve
•Not absorbed orally
•Poor tissue penetration as hydrophilic
•Eliminated by renal filtration.
describe the activity and pharmacology of nitrofurans
•Synthetic relatively toxic
describe the activity and pharmacology of tetracyclines
•Many Gram +ve and –ve.
•Atypical bacteria Ricketssia, Borellia, Chlamydia and Mycoplasma
•Can be absorbed orally but varies in group.
•Food dairy and anti-acids impair use.
•Concentrate in liver significant biliary secretion.
•Can cause GIT imbalances.
describe the activity and pharmacology of chloramphenicol
•Broad spectrum.
•Clinically ineffective against Chlamydia.
•Good distribution including CNS and eye.
•Banned from use in food animals.
describe the activity and pharmacology of macrolides
•Active against Gram +ve and good activity against anaerobic bacteria.
•High lipid solubility wide body distribution across cell barriers.
•Bacteriostatic (Erythromycin can kill at high concentrations).
•Chromosomal resistance can develop.
•Texts say all Gram –ve are resistant but not true.
describe the activity and pharmacology of lincosamides and pleuromutlins
•Primarily Gram +ve but some Gram –ve*
•Basic, lipid soluble wide distribution in body
what antibiotics affect DNA in some way?
sulphonamides/ trimethoprim (TMPS), quionolones, nitroimidazoles
Sulphonamides and Trimethoprim action
competitive inhibitors of the enzyme dihydropteroate synthetase
Inhibits dihydrofolate synthesis which is required for dna synthesis
Quinolones
Novobiocin action
Bind to and stops DNA gyrase (topoisomerase).
This enzyme packs bacterial DNA
Inhibits supercoiling of chromosome
Disrupts DNA associated processes
Nitroimidazoles action
Reduction products of imidazole group are reactive with DNA
Reactive product damages DNA
causes DNA strand breaks
what ab affect rna synthesis
Rifampicin
action of rifampicin
Inhibits DNA dependant RNA polymerase activity
Blocks initiation of protein synthesis
what are cationic Antimicrobial peptides
for Topical application
Interact with membranes and disrupt, because the charge dynamics of
the bacterial membrane differ from that of the host.
Product licenced for dermatology (ears)*
Lipids/charge properties of host vs bacterial membrane allow for distinction
why should cationic antimicrobial peptides be used topically
at high concs it can still be some interaction with with other membranes.
So they tend to be used topically where you can
get to high concentration without really interacting with sensitive cells.
How does the activity of Nitroimidazoles depend on the infection site?
Nitroimidazoles require reduction under anaerobic conditions to form toxic intermediates. (active form
And that active form reacts with the DNA and it causes DNA damage.
What is required for the drug uptake of Aminoglycosides?
Aminoglycosides require aerobic respiration and a functional electron transport chain.
the electron transport system, which is switched on in the bacteria during aerobic growth, is required to take the drug into the bacteria, where the drug can then interact with its normal target of the ribosome.
what other factors can affect ab activity
pH
binding or proteins
degradation,
secretion.
This can inactivate or reduce activity but in some cases for example excretion in urine can increase local concentration
Other considerations for success
Time dependant activity:
Most significant factor:
The time that the serum concentration exceeds the MIC.
Increasing the serum concentration above the MIC for these will not increase killing.
Concentration dependant activity:
Most significant factor:
Killing increases with increased concentration
Requires high concentrations at drug binding site to be effective.
Not always beneficial to maintain this level between doses
The catch:
The longer the antibiotics is present and the higher the risk of selection for antibiotic resistance (next session)