Assays that employ PERMANGANOMETRY
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Hydrogen Peroxide (H2O2)
Cherry Juice for Malic Acid (C4H6O5)
Sodium Nitrite (NaNO2)
Assays that employ
PERMANGANOMETRY 3
Indirect Titration (with Preliminary Treatment)
Residual Titration
Other Assays of Permanganometry (2 categories)
• Precipitated calcium carbonate
• Precipitated manganese dioxide
• Dibasic calcium phosphate
• Titanium dioxide (with blank determination)
Other Assays of Permanganometry 4
Indirect Titration (with Preliminary Treatment)
• Potassium permanganate tablets
• Lead oxide (with preliminary treatment)
Other Assays of Permanganometry 2
Residual Titration
Direct Titration
Hydrogen Peroxide (H2O2) Type of titration:
Redox reaction
Hydrogen Peroxide (H2O2) Type:
H2O2
Hydrogen Peroxide (H2O2) Reducing agent (analyte)
KMnO4
Hydrogen Peroxide (H2O2) Oxidizing agent (titrant, volumetric solution)
H2SO4
Hydrogen Peroxide (H2O2) Acidifying agent, prevents formation of MnO2
3H2SO4 + 5H2O2 + 2KMnO4 → 2MnSO4 + K2SO4 + 5O2↑ + 8H2O
Hydrogen Peroxide (H2O2) Chemical reaction:
2; 2e-
Hydrogen Peroxide (H2O2) Factor (of analyte): because in the half-equation reaction of peroxide as a reducing agent in
acidic medium, it lost a total of ?
H2O2 → O2 + 2H+ + 2e-
iodine flask
suitable flask Hydrogen Peroxide (H2O2)
colorless to pale pink
Hydrogen Peroxide (H2O2) from _ → ?
ammonium hydroxide
alkalinize the solution and keep the calcium oxalate insoluble
filtration
filters off yung mga di nagreact
mallic acid
_ → calcium malate → caoxalate
calcium malate
mallic acid → _→ caoxalate
calcium oxalate
mallic acid → calcium malate → _
Indirect Titration
Cherry Juice for Malic Acid (C4H6O5) Type of titration:
Redox reaction
Cherry Juice for Malic Acid (C4H6O5) Type:
C4H6O5 (Malic acid)
Cherry Juice for Malic Acid (C4H6O5) Reducing agent (analyte)
KMnO4:
Cherry Juice for Malic Acid (C4H6O5) Oxidizing agent (titrant, volumetric solution)
H2SO4:
Cherry Juice for Malic Acid (C4H6O5) Acidifying agent, prevents formation of MnO2
CaCO3
Cherry Juice for Malic Acid (C4H6O5) reacts with C4H6O5 to form calcium malate (CaC2H6O5); its CO32- turned to CO2 gas (which was facilitated by heating)
Cherry Juice for Malic Acid (C4H6O5)
_ reacts with C4H6O5 to form calcium malate (CaC2H6O5); its CO32- turned to CO2 gas (which was facilitated by heating)
calcium malate (CaC2H6O5)
Cherry Juice for Malic Acid (C4H6O5) reacts with C4H6O5 to form _; its CO32- turned to CO2 gas (which was facilitated by heating)
CO2 gas
Cherry Juice for Malic Acid (C4H6O5) reacts with C4H6O5 to form calcium malate (CaC2H6O5); its CO3
2- turned to _
(which was facilitated by heating)
(NH4)2(C2O4)
Cherry Juice for Malic Acid (C4H6O5) converts CaC2H6O5 to calcium oxalate (CaC2O4) crystals (the form of malic acid titrated by KMnO4, instead of malic acid itself)
CaC2H6O5
Cherry Juice for Malic Acid (C4H6O5) converts _ to calcium oxalate (CaC2O4) crystals (the form of malic acid titrated by KMnO4, instead of malic acid itself)
calcium oxalate (CaC2O4) crystals
Cherry Juice for Malic Acid (C4H6O5) converts CaC2H6O5 to _ (the form of malic acid titrated by KMnO4, instead of malic acid itself)
NH4OH
Cherry Juice for Malic Acid (C4H6O5) maintains CaC2O4 insoluble by providing NH4+ to keep the reaction forward
malic acid
_is converted to calcium malate, and then finally to calcium oxalate
calcium malate
malic acid is converted to _, and then finally to calcium oxalate
calcium oxalate
malic acid is converted to calcium malate, and then finally to _
Cherry Juice for Malic Acid (C4H6O5)
colorless → pink
Sodium Nitrite (NaNO2)
contains 97.0-101.0% of NaNO2, calculated on the dried basis (USP)
(Double) Residual Titration
Sodium Nitrite (NaNO2) [Type of titration:
Redox reaction
Sodium Nitrite (NaNO2) Type:
NaNO2:
Sodium Nitrite (NaNO2) Reducing agent (analyte)
KMnO4:
Sodium Nitrite (NaNO2) Oxidizing agent (excess titrant, volumetric solution)
H2SO4:
Sodium Nitrite (NaNO2) Acidifying agent (prevents formation of MnO2)
H2C2O4:
Sodium Nitrite (NaNO2) Reducing agent (backtitrant)
3H2SO4 + 5NaNO2 + 2KMnO4 → 2MnSO4 + 5Na2NO3 + K2SO4 + 3H2O
3H2SO4 + 5H2C2O4.2H2O + 2KMnO4 → 2MnSO4 + 10CO2↑ + K2SO4 + 18H2O
Sodium Nitrite (NaNO2) Chemical reactions
2
Sodium Nitrite (NaNO2) Factor (of analyte): _ (because in the half-equation reaction of nitrite as a reducing agent in
acidic medium, it lost a total of 2e-.
%P formula
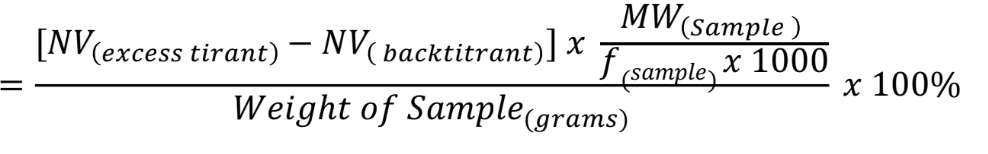
Indirect Titration (with Preliminary Treatment)
Residual Titration
Other Assays of Permanganometry 2
• Precipitated calcium carbonate
• Precipitated manganese dioxide
• Dibasic calcium phosphate
• Titanium dioxide (with blank determination)
Indirect Titration (with Preliminary Treatment) 4
• Potassium permanganate tablets
• Lead oxide (with preliminary treatment)
Residual Titration 2