Lab 1: Melting Point and Melting Point Depression
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
melting point
the temperature at which a solid changes into a liquid at standard atmospheric pressure
for a pure compound, it is the temp at which the vp of the solid and liquid are equal
thermal energy becomes sufficient to overcome the IMFs holding the particles in a rigid structure
melting point depression
the phenomenon where the melting point of a substance decreases when it is impure or a mixture
impurity must be soluble to lower mp
eutectic point
the specific composition and temperature at which a mixture of two or more substances has the lowest mp
why is melting point determined?
if the compound is known, it helps to characterize the sample
if the compound is new, it is recorded to allow future characterization by others
the range of the mp is indicative of the purity of the compound
how is melting point determined?
IMFs
order of IMF strength
London Dispersion Forces
Pi-Pi Stacking
Dipole-Dipole
Hydrogen Bonding
Ion-Dipole
Ionic Attractive Forces
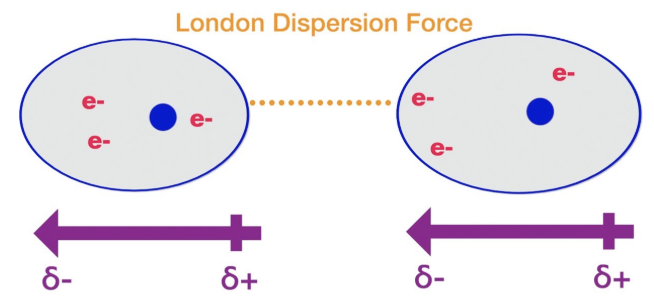
London Dispersion Forces (Van der Waals Forces)
Organic molecules that contain only carbon and hydrogen (hydrocarbons) are weakly attracted to each other by London Dispersion forces
Increase as molecular size increases
The larger the molecule, the greater the attractive force for neighboring molecules and the greater the energy required to get two molecules to move apart
Temporary
Occur due to random movement of electrons
Strength increases with size, surface area, and number of electrons
Organic hydrocarbons
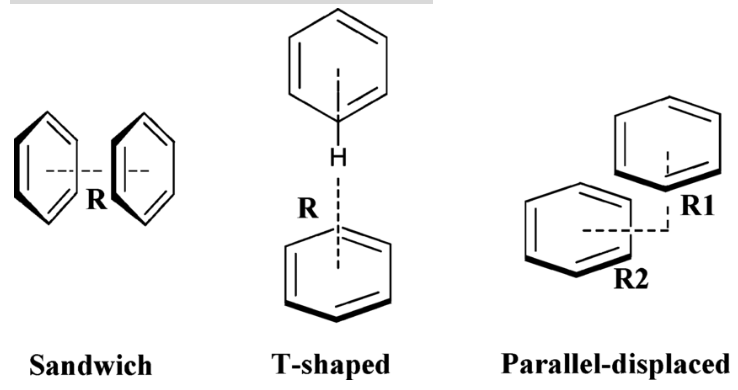
Pi-Pi Stacking
Type of noncovalent interaction that occurs between aromatic rings due to the overlap of their pi bonds
Dipole moment between two aromatic rings
Generally considered weak to moderate in terms of strength depending on other IMFs
Important in DNA structure
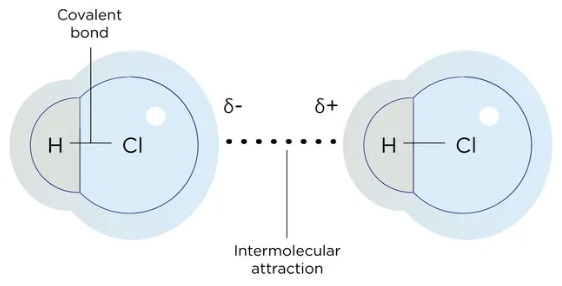
Dipole-Dipole
The attractive forces between molecules increases when functional groups containing electronegative atoms, such as chlorine, oxygen, and nitrogen are present because these atoms are more electronegative than carbon
Electronegative atoms pull electrons toward themselves, making their end of the bond slightly negatively charged (δ-) and leaving the carbon slightly positively charged (δ+)
Electronegative atoms pull electrons towards themselves
Permanent dipole
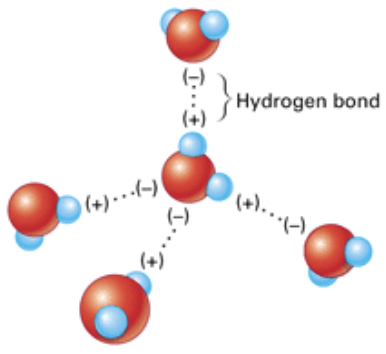
Hydrogen Bonding
Occurs with organic molecules containing O–H groups (alcohols and carboxylic acids) or N–H groups (amines or amides)
The hydrogen in these groups is attracted to the unshared pair of electrons on the O or N of another molecule, forming a hydrogen bond, often symbolized by a dashed line
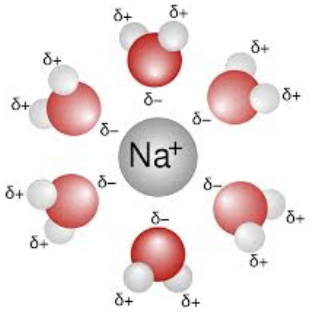
Ion-Dipole
Electrostatic attractions between an ion and a polar molecule, where the oppositely charged ends of the ion and the dipole are attracted to each other
Ionic Attractive Forces
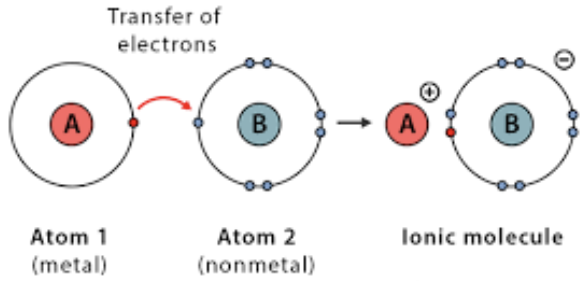
The electrostatic force that occurs when oppositely charged ions are attracted to each other, forming an ionic bond
Form when a metal atom (which tends to lose electrons to become a positive ion, or cation) and a nonmetal atom (which tends to gain electrons to become a negative ion, or anion) transfer electrons
The strong, permament electrostatic force of attraction that hold together oppositely charged molecules
Usually solids with high melting points due to the strong attractive forces between them
what type of compounds have really high melting points?
ionic (salts)
what effect would poor heat circulation have on the observed mp?
Inaccurate observed melting point
Potentially higher or lower than the true value
Causes non-uniform heating and poor heat transfer
Uneven melting and sample decomposition
Three test tubes, labels A, B, and C, contain substances with approximately the same melting points. How could you prove that the test tubes contain three different chemical compounds?
Mixed melting point test
Mixing two substances and performing melting point will lead to a broader and lower range than their pure melting points
Spectroscopy
NMR
IR
MS
Chromotography
TLC
how much does a small impurity (1-2%) depress the mp?
1-2°C
how does a 10% impurity change the mp?
typically depresses the mp by 5-10°C, often also broadening the range
You suspect that an unknown is acetanilide (mp 113.5°C-114.0°C). Give a qualitative estimation of the melting point when the acetanilide is mixed with 10% by weight of naphthalene.
105-110°C
You have an unknown with an observed melting point of 90.1°C–93.5°C. Is your unknown compound A with a reported melting point of 95.5°C–96.0°C, or compound B with a reported melting point of 90.5°C–91.0°C? Explain.
Compound A because the temperature at which the compound is 95% melted is higher than the final melting point temperature of compound B
An unknown compound is suspected to be acetanilide (mp 113.5°C–114°C). What would happen to the melting point if this unknown were mixed with (a) an equal quantity of pure acetanilide? (b) an equal quantity of benzoic acid.
(a) If the sample were mixed with pure acetanilide, the melting point would remain the same
(b) If the sample were mixed with benzoic acid (mp 122°C), the melting point of the mixture would be below that of acetanilide and the mixture would melt over a broadened, large, depressed range, perhaps 100-105°C or lower