Lecture 11: biosynthesis of triglycerides and cholestrol
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
27 Terms
Origins of glycerol
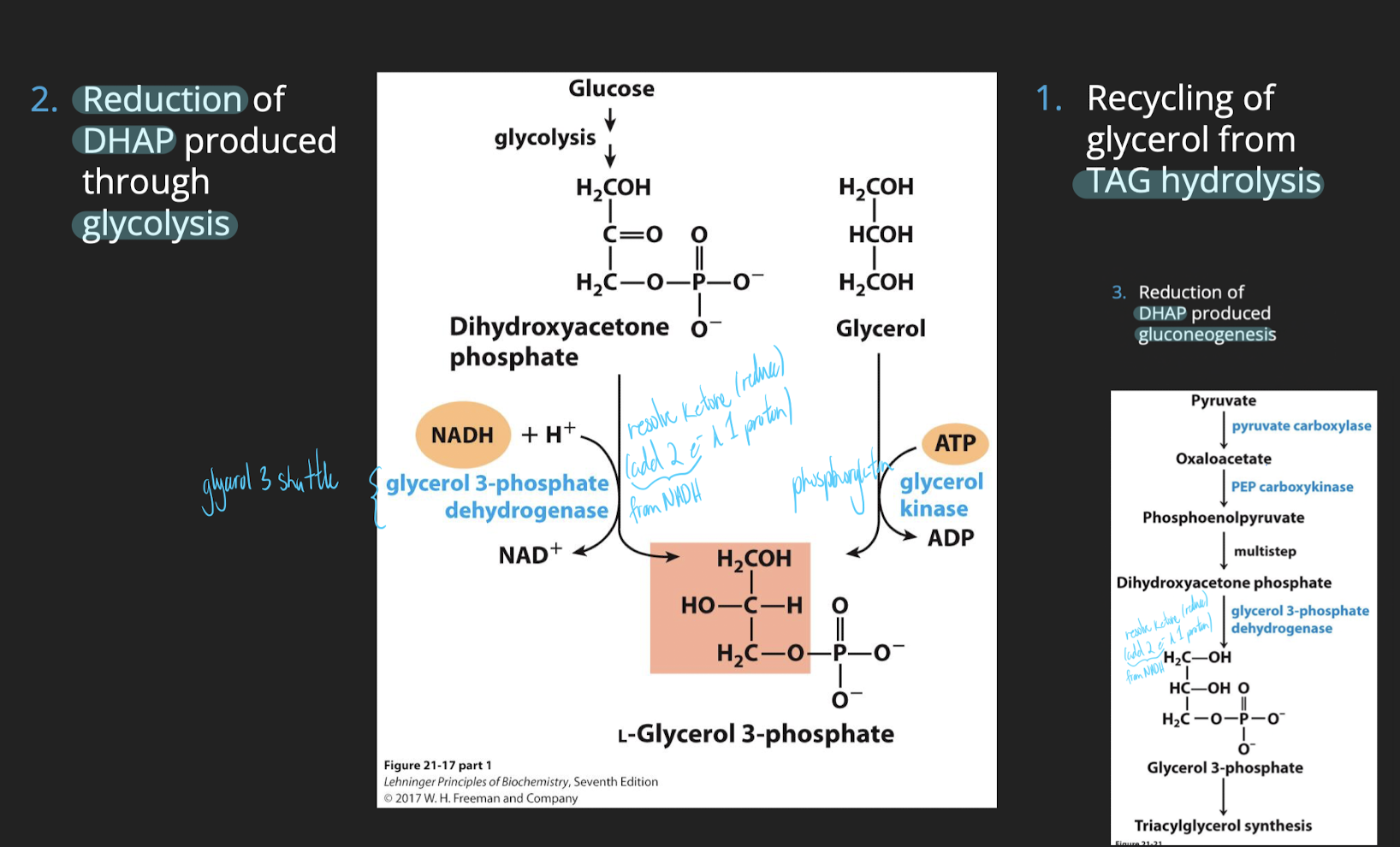
the limiting substrate during TAG synthesis is the glycerol backbone.
More precisely, TAG synthesis begins with a molecule of glycerol-3-phosphate.
Sources of glycerol-3-phosphate
Recycling of the glycerol produced during lipolysis of TAG
in adipose tissue, FA will be released from TAG
Reduction of dihydroxyacetone phosphate (DHAP) produced during glycolysis.
1 molecule of glucose goes into make DHAP
Reduction of dihydroxyacetone phosphate (DHAP) produced during glyceroneogenesis.
Using gluconeogenesis to produce glycerol
Lipolysis
TAG and cleave FA to release them so it can be used as a source of energy
gluconeogenesis
formation of new glycerol-3-phosphate from anythin other than glucose
Origins of glycerol: Adipose tissues
Adipocytes lack glycerol kinase and therefore, they rely on pathways involving DHAP (glycolysis or glyceroneogenesis)
Glycerol produced by the b-oxidation is returned to the liver through the blood stream to be reused (using Glycerol kinase).
Most of the glycerol 3-phosphate is produced through glyceroneogenesis.
adipose tissue cant regulate own cholesterol
glycerol being produced by adipose tissue can’t be converted to glycerol phosphate
Origins of glycerol: Liver
Glycerol is acquired using all three mechanisms
adipose, DHAP glycolysis & glyconeogenesis
TAG synthesis
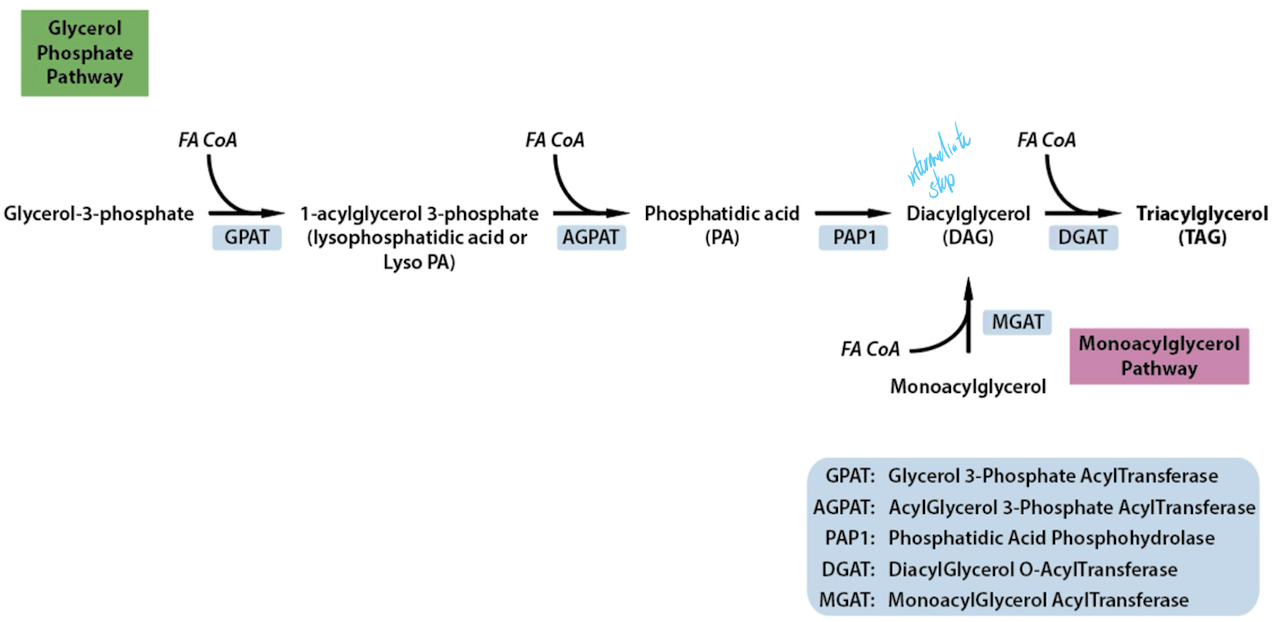
Glycerol phosphate pathway: Liver, adipose tissues and intestine
Monoacylglycerol pathway: Specific to the intestine
Glycerol phosphate pathway- DAG formation
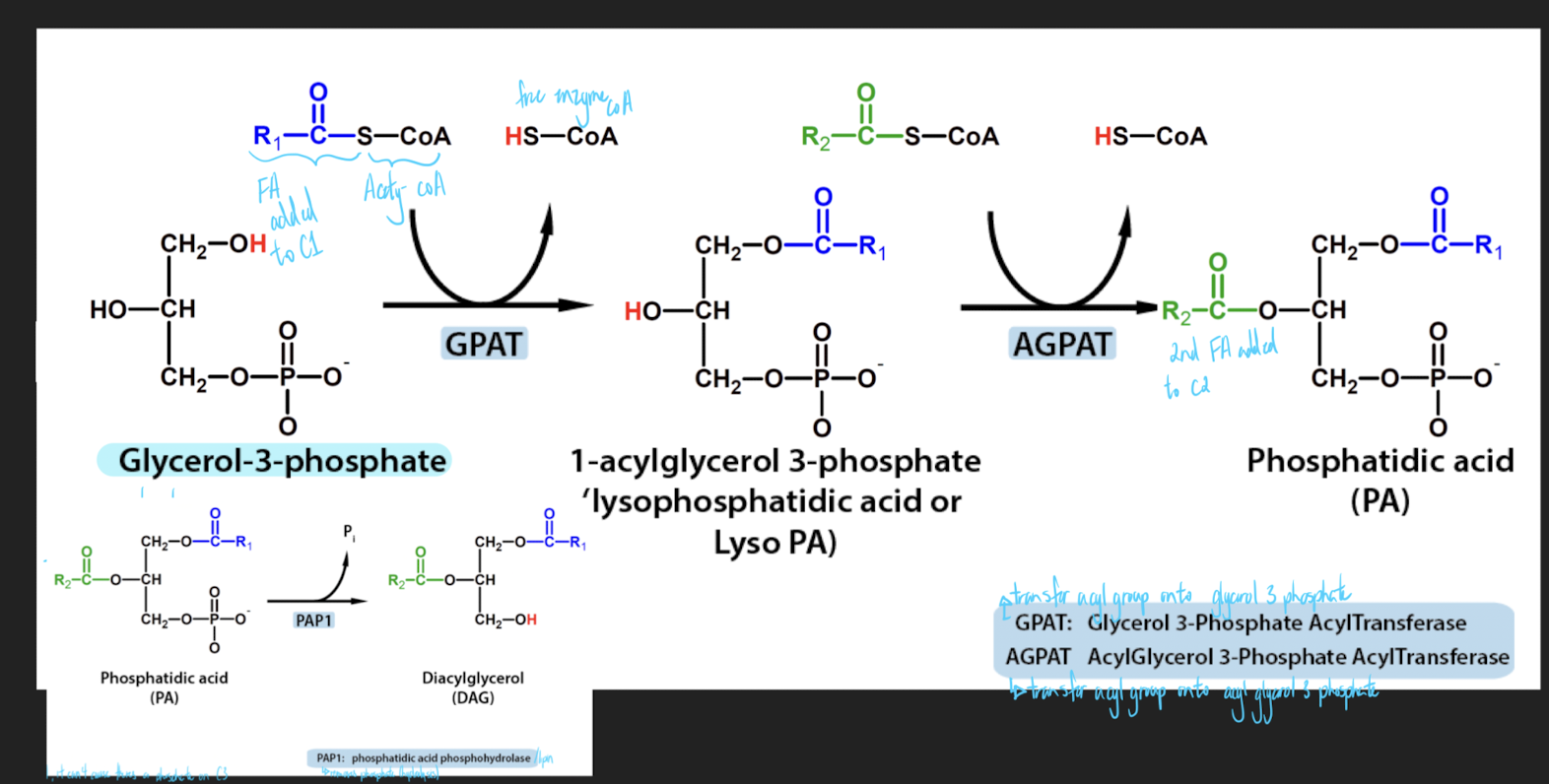
Actions of GPAT and AGPAT
use 2 enzymes to add a chain to carbon 1 and carbon 2
PAP enzymes are also called Lipin (family of three proteins)
if you want to add a 3rd FA, it can’t because theres a phosphate on C3
Monoacylglycerol pathway- Source of monoacylglycerol
Action of pancreatic lipase leads to the production of free fatty acids (FFA) and 2- monoacylglycerol
pancreatic lipase hydrolyzes FA on carbons 1 and 3 (on edge therefore easy to access)
Short and medium-chain FAs (as well as glycerol) pass directly from the intestine to the lymphatic system (not shown)
mechanism: flip flop
Free fatty acids can be reassembled into TAG through the glycerol 3-phosphate pathway (also called, the Phosphatidic acid pathway)
2-monoacylglycerol molecules enter the enterocyte and then, are converted to TAG through the monoacylglycerol pathway before being released into the lymphatic system
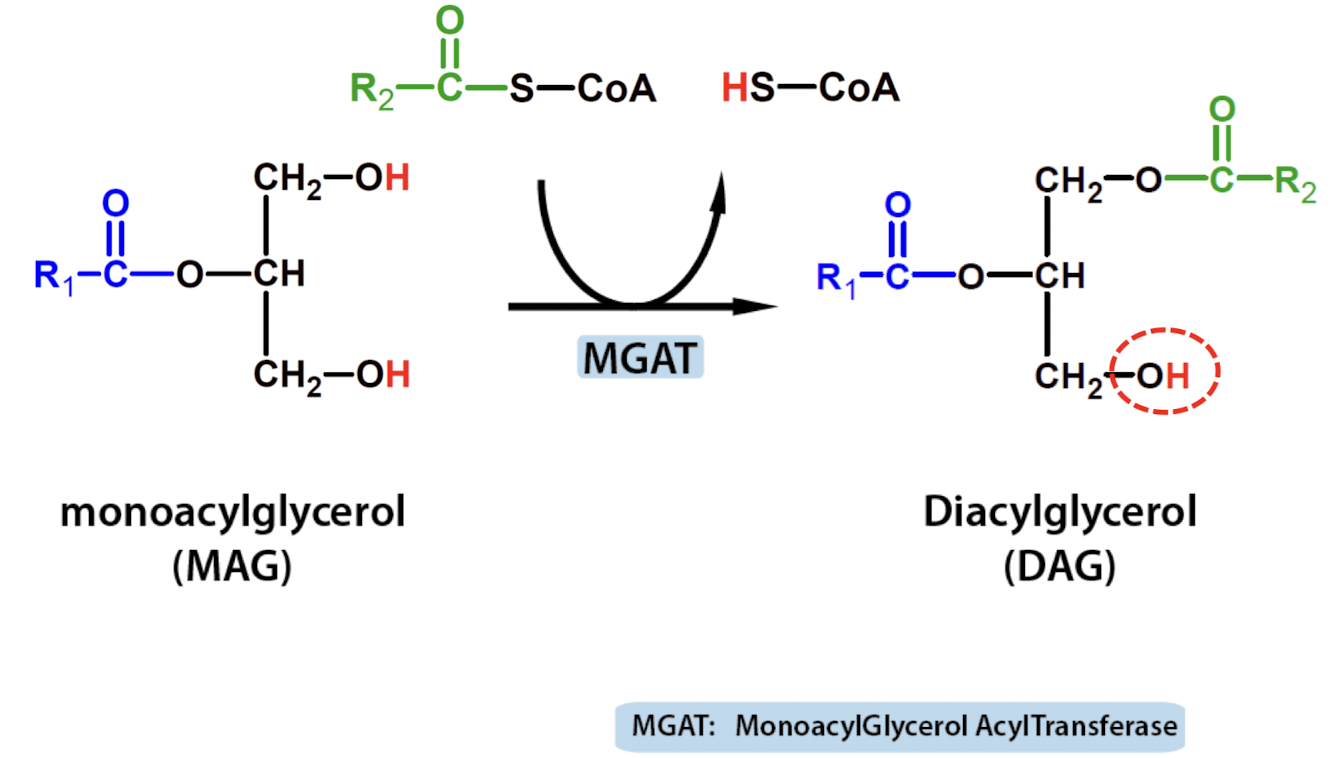
Monoacylglycerol pathway
deals with TAGs coming from diet
no dephosphorylation
Monoacylglycerol pathway-DAG formation
Action of MGAT in the intestinal cells
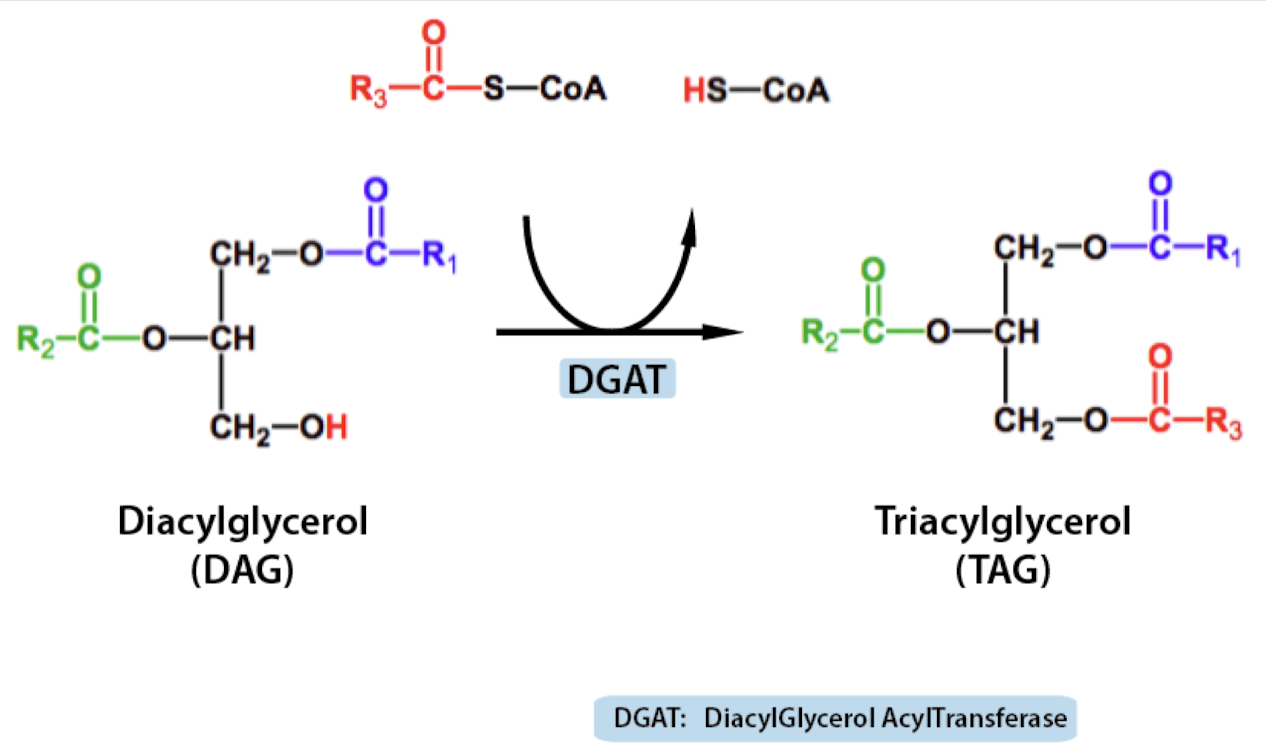
TAG formation
uses DGAT to turn DAG to TAG
Localization of TAG biosynthesis machinery
Endoplasmic reticulum (ER) and the mitochondria outer membrane
enzymes are located at the mitochondria and the ER facing the outside (on surface)
lots of different enzymes
TAG synthesis
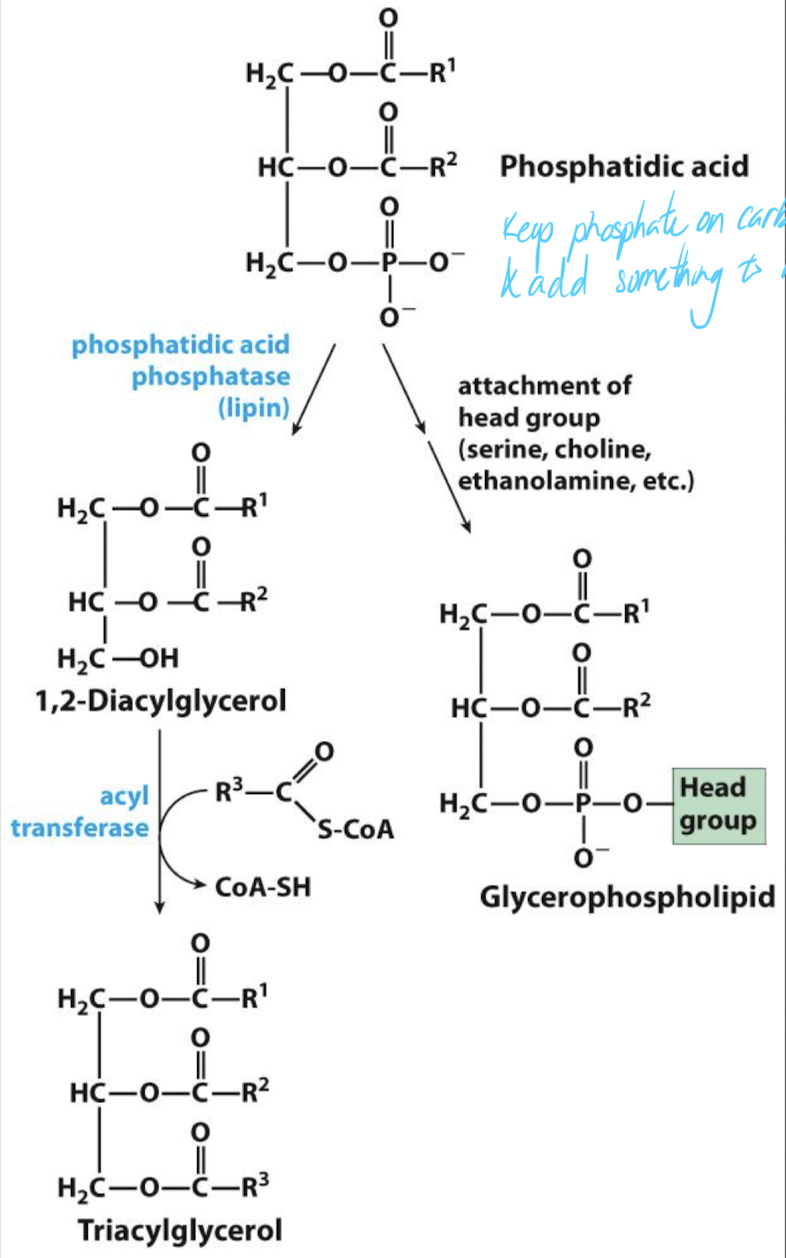
Phosphatidic acid (PA) is a key intermediate for TAG synthesis but can also be used in the biosynthesis of glycerophospholipids.
keep phosphate on carbon #3 and add something to it
What is the fate of TAGs?
1. Storage in Lipids droplets (LDs)
Storage of energy
LDs are highly hydrophobic and therefore they remain in the cells.
FA can also be released from LD and access the bloodstream or travel to mitochondria to be used as immediate energy source (b-oxidation).
These work on the surface of the oil droplet
ATGL = Adipose TriGlyceride Lipase
HSL = Hormone sensitive lipase
MGL = Monoacyglycerol lipase
Release of FA is controlled by hormones
glucagon = decrease glucose= release energy
glucagon stimulates release of FA
increase lipolysis ((release of FA from TAG)
Redistribution of TAG throughout the body
Chylomicrons (intestine)
Very-Low-Density Lipoprotein (liver)’
Coat TAG in phospholipids so that it can be distributed throughout the body
What is the effector of glucagon?
pKa
it phosphorylates perilipin CGi-58 is released and attacks ATGL, ATGL releases FA
Biological functions of cholesterol
structural component of cell membranes, particularly the plasma membranes
constituent of lipoproteins
precursor for bile acids and steroid hormones
Cholesterol Biosynthesis
Around 50-80% of the total cholesterol found in your body is coming from de novo biosynthesis
The other 20-50% is from your diet and the recycled cholesterol (surplus is excreated, picked up and restored)
Roughly 80% of total daily cholesterol production occurs in the liver and intestine.
Cholesterol is also produced in the adrenal glands, and reproductive organs.
All of cholesterol’s carbon atoms are derived from acetate
Where does acetate come from?
Acetyl-CoA
Tricarboxylate transport system
Too much ATP= slow down TCA cycle
acetyl coa is diverted to something else (FA synthesis or cholesterol synthesis)
Three stages of cholesterol synthesis in the liver
condensation of acetate to form mevalonate intermediates (C6 unit)
polymerization of mevalonate to form squalene (C30 unit)
cyclization of squalene and further modifications to form cholesterol (C27 unit)
Cholesterol synthesis- stage 1
Condensation
from acetyl-CoA (C2) to mevalonate (C6)
HMG-CoA reductase catalyzes the rate limiting step of cholesterol biosynthesis.
HMG-CoA reductase is an integral membrane protein
HMG-CoA reductase can be found in the ER and peroxisome
from mevalonate (C6) to dimethylallyl pyrophosphate (C5) (Part 1)
lose 1 carbon
from mevalonate (C6) to dimethylallyl pyrophosphate (C5) (Part 2)
adding phosphate facilitate decatrboxylation
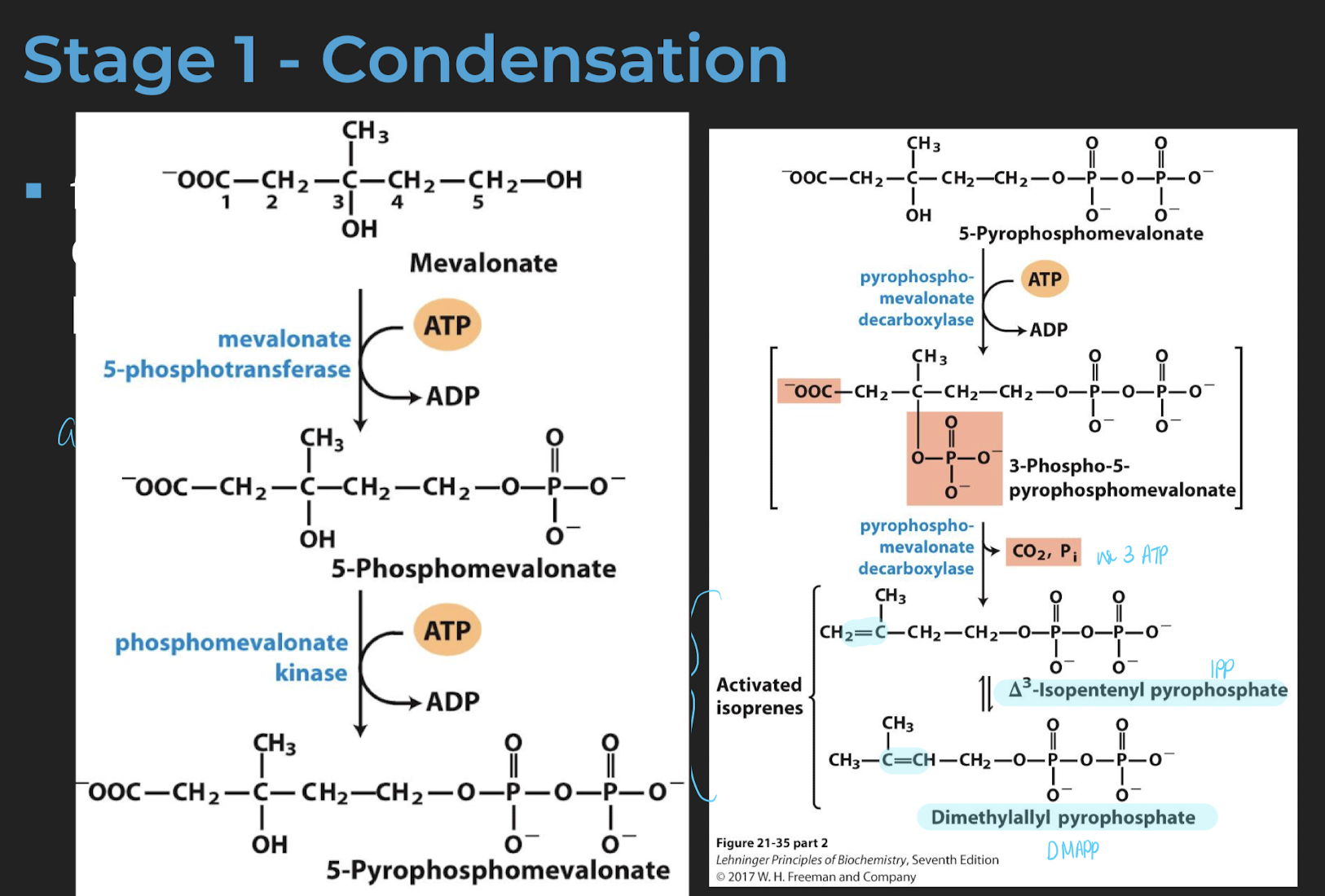
Isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate are isoprenoids
Isoprenoids are the oldest know biomolecules.
Recovered from sediments which were roughly 2.5 billion years old.
Family of around 30,000 known compounds
Definition of isoprenoids: any compound biosynthesized from or containing isoprene units
Cholesterol synthesis- stage 2
Polymerization of 3 isoprenoids (C5) to form farnesyl pyrophosphate (C15)
Reactions 1 and 2 are catalyzed by prenyltransferase
Nucleophilic substitutions
Final product is Farnesyl pyrophosphate (C15)
use 1 dimethylallyl pyrophosphate, 1 isopentenyl pyrophosphate
Polymerization of farnesyl pyrophosphate (C15) to form. squalene (C30)
Reactions 3 is catalyzed by squalene synthase
This enzyme is located in the ER membrane
Join two molecules of Farnesyl pyrophosphate in a head-to-head conformation
Final product is Squalene
use 1 NAPDH
Cholesterol synthesis- stage 3
Cyclization
From squalene (C30) to cholesterol (C27)
Cyclization of squalene to form lanosterol (C30)
Synthesis of cholesterol (C27) from lanosterol (C30)
Precursors
Cholesterol and isoprenoids are precursors of other compounds
Summary- The cholesterol biosynthesis pathway
Building block for de novo cholesterol biosynthesis is acetyl-CoA
HMG-CoA reductase catalyzes the rate limiting step
Important intermediates are HMG-CoA, mevalonate, activated isoprenoids (IPP and DMAPP)
Enzymes are located in peroxisome (pre-squalene) and ER