Aqa alevel chemistry atomic structure
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
What are subatomic particles, mass and charges?
Proton: 1 +1
Neutron 1 0
Electron 1/1840 -1
What is the mass number?
The total number of protons and neutrons in the nucleus of an atom
What is the atomic number?
The number of protons in the nucleus of an atom
How are ions formed?
when atoms gain or lose electrons
What are isotopes?
Isotopes of an element are atoms with the same number of protons but different numbers of neutrons.
they have slightly different psychical properties because the mass are different due to different number of neutrons
eg rates of diffusion, density etc
Dalton's model
Described atoms as solid spheres and that different spheres made up different elements
Thompson's model
Concluded that an atom must contain even smaller, negatively charged particles -which are electrons in a ball of positive charge
aka plum pudding model
Rutherford's model
Gold foil experiment:
shot alpha particles (positively charged) at a thin sheet of gold.
according to plum pudding model, the alpha particles should have deflected due to ball of positive charge but instead most alpha particles passed through and only some deflected.
so Rutherford said there's a small positively charged nucleus in the middle of the atom surrounded by a cloud of negative electrons. Most of the atom is empty space
Bohr's model
If electrons were in a cloud around the nucleus of an atom they would spiral down into the nucleus causing the atom to collapse
Bohr's principles
1 Electrons only exist in fixed shells are not anywhere in between
2 Each shell has a fixed energy
3 When an electron moves between shells electromagnetic radiation is emitted or absorbed
4 Because the energy of shells is fixed, the radiation will have a fixed frequency
What is relative atomic mass?
The average mass of an atom on a scale where an atom of carbon-12 is exactly 12
What is relative isotopic mass?
The average mass of an atom of an isotope on a scale where an atom of carbon-12 is exactly 12
What is relative molecular mass?
The average mass of a molecule on a scale where an atom of carbon-12 is exactly 12
What is relative formula mass?
The average mass of a formula unit on a scale where an atom of carbon-12 is exactly 12
What is the mass spectrometer?
Gives you information about the relative atomic mass of an element and the relative abundance of its isotopes
What are the 5 stages of mass spectronomy?
Ionisation
Acceleration
Ion Drift
Detection
Data Analysis
What happens in ionisation?
The sample is ionised before entering the mass spectrometer
electrospray ionisation - the sample is dissolved in a solvent and pushed through a fine hypodermic needle at a high pressure with a high voltage attached, causing each particle to gain an H+ ion. The solvent is then removed leaving a gas made up of positive ions
electron impact - the sample is vaporised and an electron gun fires high energy electrons at it, knocking off on electron off each particle so they become +1 ions
What happens in acceleration?
Positive ions are accelerated by an electric field which gives the same kinetic energy to all ions. The lighter ions accelerate more than heavy ions
What happens in ion drift?
Ions enter a region wiht no electric field. They drift at the same speed they left the electric field so lighter ions drift at higher speeds
What happens in detection?
The detectors detect the current created when the positive ions hit the negatively charged detector plate
What happens during data analysis?
The signal from the detector passes to a computer which generates a mass spectrum
What is a mass spectrum?
mass/charge plotted against % abundance graph
How do you calculate relative atomic mass?
Sum of (isotope abundance x isotope mass number) / sum of abundances of all the isotopes
How do you calculate molecular mass?
Mass/charge ratio (m/z) is equal to the mr
What are the sub shells?
Within a shell, orbitals of the same type are grouped together as sub-shells
s,p,d
1s2 2s2 2p6 3s2 3p6 4s2 3d10
Rules for electron configuration
1. Electrons fill up the lowest energy sub shell first
2. Electrons fill sub shells singly before sharing
3. For ions just add/remove electrons
Electron configuration of transition metals
Chromium:
1s2 2s2 2p6 3s2 3ps 3d5 4s1
copper:
1s2 2s2 2p6 3s2 3p6 3d10 4s1
all transition metals lose their 4s electrons before their 3d ones
What is the first ionistaion energy?
The energy needed to remove one electron from one mole of an atom to form one mole of gaseous ion
O(g) -> O+(g) + e-
What are the factors affecting ionisation energy?
Nuclear charge
More protons-more positive nucleus-stronger attraction for e-
Distance from the nucleus
The longer distance of nucleus to e-, the weaker the attraction
Shielding
the more e- between outer e- and nucleus, the weaker the attraction
What is the second ionisation energy?
The energy needed to remove an electron from one mole of a 1+ ion
O+(g)-> O2+(g) + e-
needs more energy than first ionistaion because the e- is removed from a positive ion not an atom
What is successive ionisation?
The removal of more than 1 electron from the same atom
general equation
X(n-1)+(g) -> Xn+(g) + e-
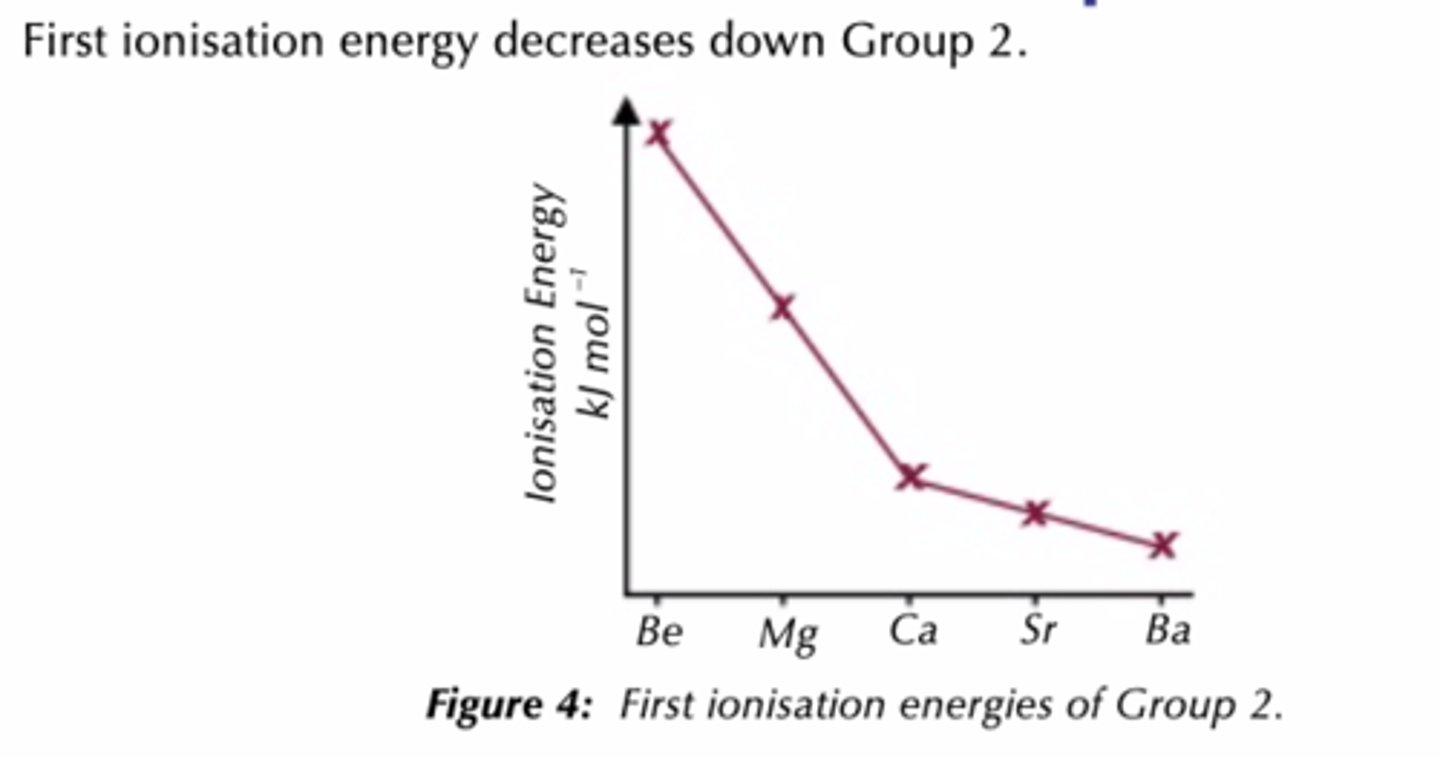
What is the ionisation trend down group 2?
Decreases as
increasing atomic radius and shielding
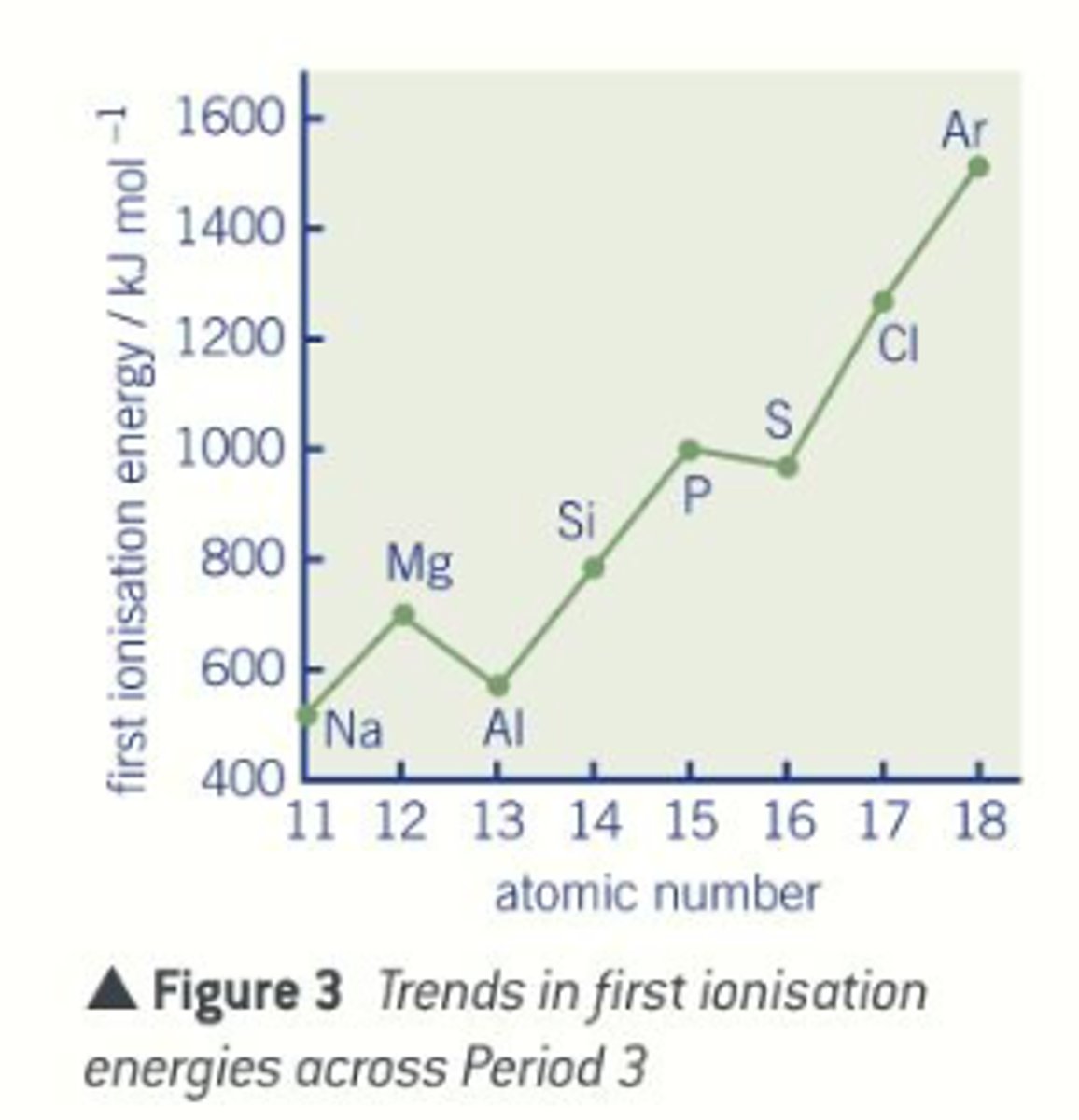
What is the ionisation trend across period 3?
Increases as
similar shielding and radius but increasing no. of protons so the attraction between nucleus and e- is stronger
1. Aluminium deviates from the trend as outer e- is in the 3p orbital rather than 3s. E- in the 3p orbital lost more easily than those in 3s orbital, as further away from nucleus, hence lower first ionisation energy
2. Sulphur deviates from trend due to electron repulsion. Both p and s have e- removed from 3p orbital but the e- in sulphur is being removed from a pair of e- whereas in phosphorus the e- is single. Therefore the e- is easier to remove due to electron repulsion between the pair