Organic Chemistry I
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
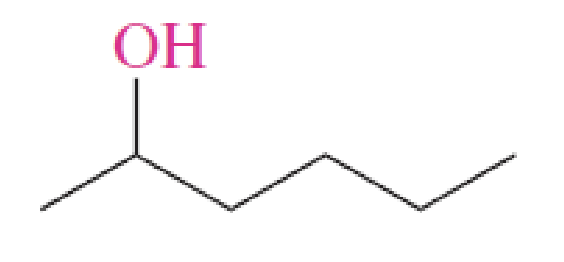
Alcohols
molecules which bare a standalone hydroxy (-OH) group
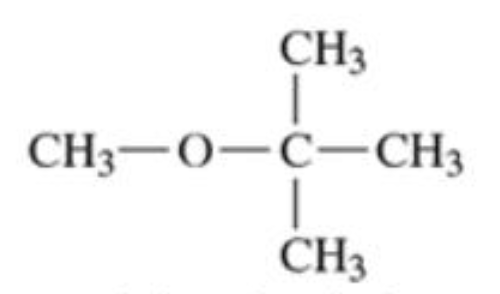
Ethers
molecules which contain two alkyl groups bonded to an oxygen (R-O-R’)
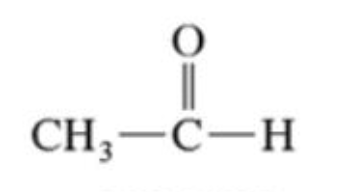
Aldehydes
has one alkyl group and a carbonyl group (C=O) bonded to at least one hydrogen atom.
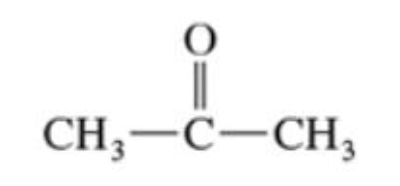
Ketones
have two alkyl groups bonded to the carbonyl group (C=O)
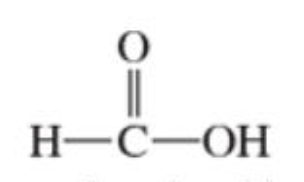
Carboxylic acids
contains a carboxylic group (-COOH), its a combination of a carbonyl and a hydroxy group
Amines
alkylated derivatives of ammonia
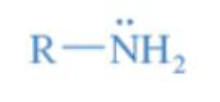
Amides
derivatives of carboxylic acids made by reacting -COOH with an amine, the combination of a carbonyl and an amine
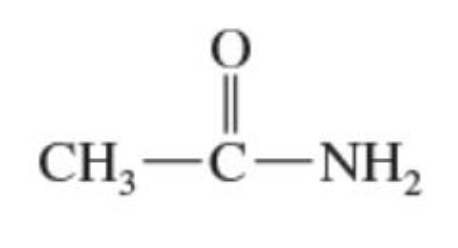
Nitriles
contains R-C≡N group (sp hybridized)
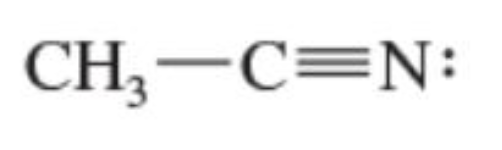
Arrhenius Acid
a molecular compound which dissociates in water to give hydronium ions
Arrhenius base
a molecular compound which dissociates in water to give hydroxide ions
Bronsted-Lowry acid
any molecule which can be a proton donor
Bronsted-Lowry base
any molecule which can be a proton acceptor
Lewis Acid
electron acceptor (electrophile)
Lewis base
electron donor (nucleophile)
Hydrocarbons
molecules containing only hydrogen and carbon molecules
Classes of hydrocarbons
aliphatic and aromatic
Types of aliphatic hydrocarbons
alkanes, alkenes, and alkynes
Alkanes
all single bonds
Alkenes
at least one double bond
Alkynes
at least one triple bond
Benzene
single, double, single bonded carbons in a hexagon
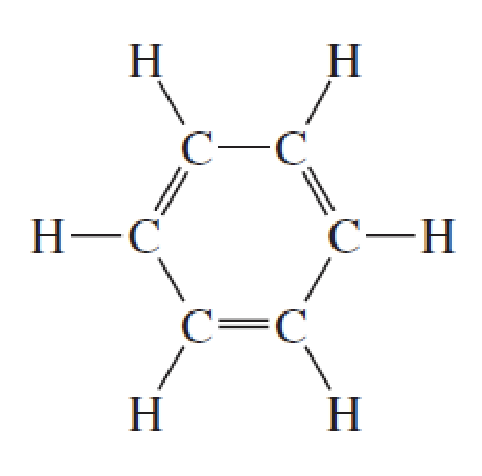
Isomers
compounds which are different but have the same molecular formula
Constitutional isomers
a type of isomer in which the molecules have different connectivity
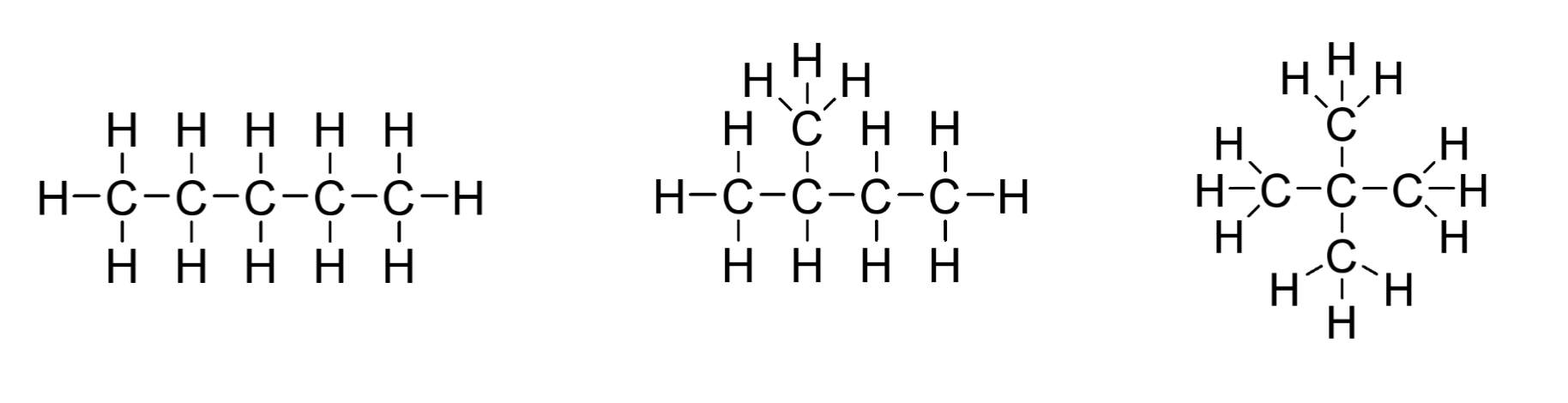
Stereoisomers
molecules which have the same molecular formula and sequence of bonded atoms but differ in the spatial orientation of atoms or groups in the molecule
Conformational isomers
a type of stereoisomer in which two identical molecules only differ in the conformation of atoms in 3D space
Categories of conformational isomers
Staggered and eclipsed
Factors affecting acid strength
Resonance, size, electronegativity, inductive effects, s-character