Cariology Lecture 1
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
Dental Caries
A biofilm-mediated, sugar-driven, multifactorial, dynamic disease that results in the physic demineralization and remineralization of dental tissues
What is the most prevalent condition world-wide?
Untreated dental caries in permanent teeth
In 2010, how many people worldwide did untreated caries affect?
2.4 billion
True or false: More individuals suffer from dental disease than any other illness known to humans.
True
What percentage of Americans have some form of dental disease?
98%
What are the goals of treatment of caries?
-Prevent new ones from forming
-Detect lesions early so that they can be treated and arrested by noninvasive means
-Educate pts on the causes of tooth decay and give them preventative tools
How do caries form?
An imbalance of demineralization and remineralization of the teeth
Oral cavity bacteria ferments carbs (sugars) into organic acids, which results in the dissolution of dental hard tissue in the case of lactic acid
What three things interact to form caries?
Teeth
Microorganisms
Saliva
Are caries preventable?
In most cases, yes
What causes demineralization?
Acids that form during the fermentation of sugars by oral bacteria
Lack of protective saliva
Tooth morphology/composition
How can you remineralize teeth?
Remove carcinogenic bacteria through effective oral hygiene
Good salivary flow
Topical fluoride
Sealants
Anticaries/remineralizing agents
Diet modifications
What types of food and drinks cause demineralization?
Acidic ones
4 typical suspects of caries development
Genetics
Bacteria
Diet (sugar)
Saliva
What is the outermost layer of the tooth composed of?
Hydroxyapatite (HAP), a crystalline calcium phosphate mineral
Chemical composition of HAP
Ca5(PO4)3(OH)
What is the hardest substance in the human body, and what does it provide?
Enamel, strength and protection
Percentage of inorganic content in enamel
96% by weight, the remainder is organic material and water
What else is in enamel besides HAP?
It is calcium deficient, so other ions like sodium, magnesium, and carbonate replace calcium and hydroxyl ions
What does carbonate do in the enamel?
Makes the tooth mineral more susceptible to acid dissolution
What does bleaching do to the teeth?
Dehydrates them
Dentin composition
Less mineral content and more organic matter (collagen)
70% inorganic
18% organic
12% water
Does dentin stop forming?
No, it continues to be formed after crown formation is complete
What is dentin?
A vital tissue under the enamel that is softer, but still protective
It is sensitive to hot and cold
Pulp composition
25% organic
75% water
What is the organic content in pulp?
Connective tissue cells (fibroblasts), fibers (collagenous), and ground substances (proteoglycans and fibronectin)
Is the pulp vascular?
Highly; arterioles and venues enter/exit through the apical foramen and accessory root canals
Is the pulp innervated?
Very; nerves follow the course of the blood vessels with extensions into the dentin
When does one need root canal therapy?
When the pulp becomes necrotic
3 pairs of glands that produce saliva
Parotid
Submandibular
Sublingual
How much saliva is secreted per day?
0.7-1.5L
What is saliva composed of?
99% water
Electrolytes and organic components like proteins, glycoproteins, and enzymes
Pellicle
A thin, bacteria-free layer covering the teeth, formed by the absorption of salivary proteins that have a high affinity for surface minerals in the tooth.
Plays an important role in protecting dental hard tissue against mechanical and chemical damage
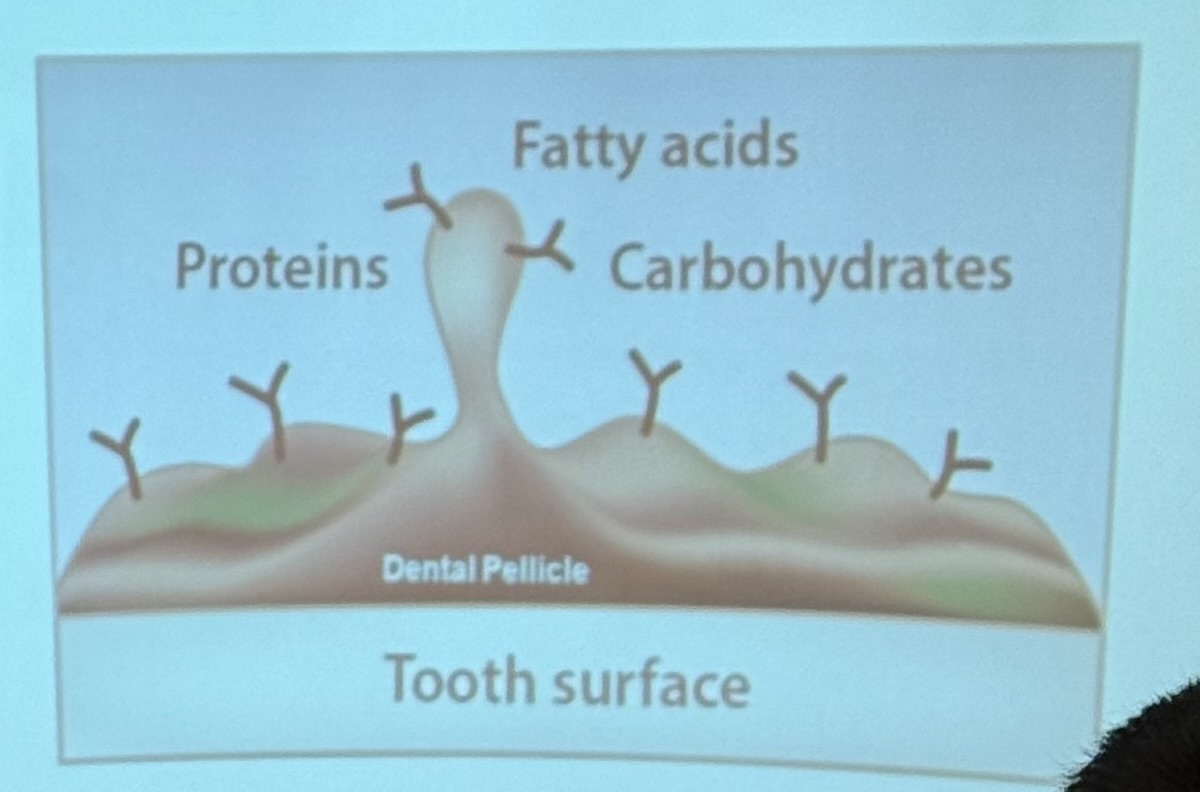
What does salivary water do?
Rinsing effect of the mouth
Solubilization of food
Facilitation of food clearance
Lubrication of oral soft tissues
Facilitation of mastication, swallowing, and speech
Functions of salivary electrolytes
Maintaining supersaturated calcium and phosphate concentrations and neutralization of acid by buffering actions
Functions of salivary organic components
Enamel pellicle formation
Mucosal coating
Antimicrobial defense
Digestive actions
Saliva’s protective role
Counteracting demineralization
Natural buffer to neutralize acids and raise pH
Supersaturation with calcium and phosphate ions to be redeposited onto the tooth surface
Amylase function
Degradation of starch
Lysozyme function
Antimicrobial activity by destruction of bacterial cell membranes
Lactoferrin function
Antimicrobial activity by high affinity for iron
Peroxidase function
Antimicrobial activity/protection against H2O2
Agglutinin function
Antimicrobial activity by agglutination of bacterial to large aggregates
Statherin function
Inhibits spontaneous precipitation
Function of antibodies
IgA/IgG, IgM inhibition of adhesion, enhancement of phagocytosis
How is biofilm (plaque) formed?
Sticky glycoproteins in saliva from foods high in fermentable carbs (glucose,sucrose,starches) adhere to the tooth surface forming a base for bacteria to attach and multiply
What does S. Mutans do?
Possesses enzyme glucosyltransferase
Forms dextran from glucose polymerized from sucrose
What is dextran?
A highly sticky substance that contributes to the formation of plaque, further trapping bacteria and acids against the tooth
It is a vicious cycle, sticky plaque gets more and more sticky
Bacteria and sugars produce…
Acid
Which bacteria metabolize the sugars through glycolysis?
S. Mutans and Lactobacilli
What is a key byproduct of glycolysis in saliva?
Lactic acid
What is the critical pH level for enamel?
5.5
What happens if the pH falls below that critical level in enamel?
The acidic environment causes the hydroxyapatite crystals to dissolve, which weakens the enamel structure
What happens when the enamel structure is weakened?
Microscopic pores form that can turn into visible lesions (cavities)
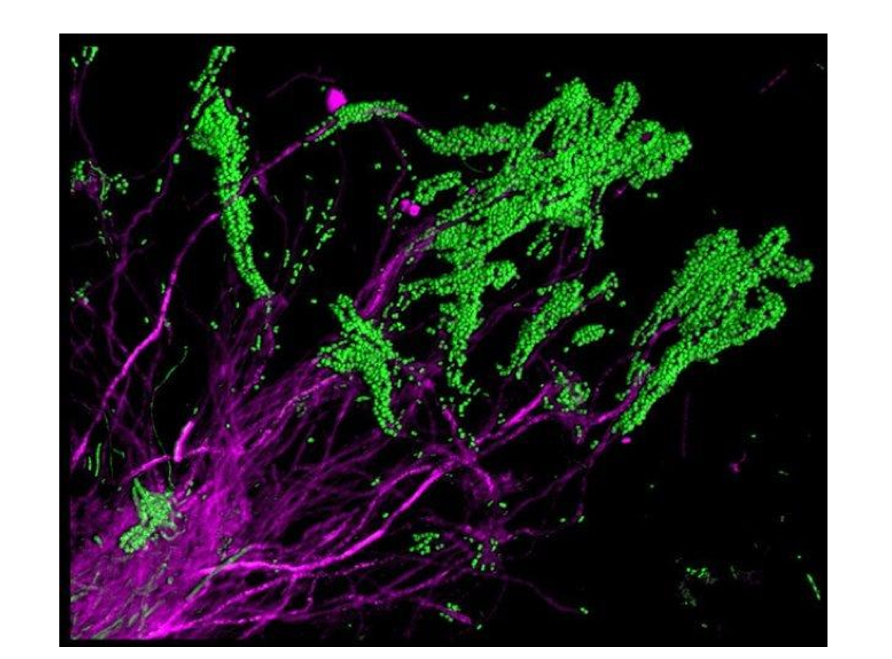
What is shown in this photo (broadly)?
Dental plaque
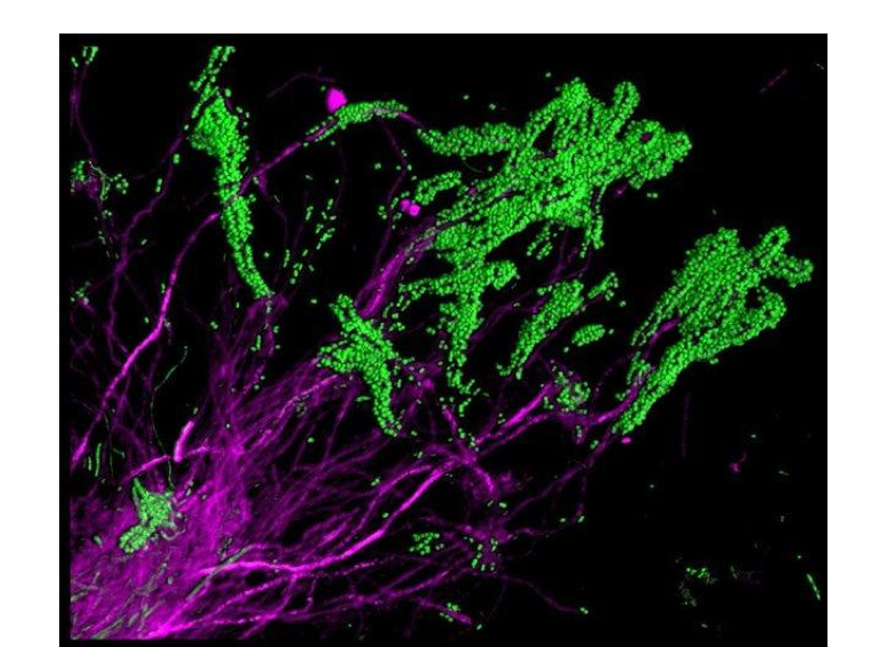
What are the green and magenta bacteria called?
Green- S. Mutans
Magenta- corynebacteria
What chemical reaction occurs when the enamel ph is less than 5.5?
Hydrogen ions react with phosphate and hydroxyl ions in the hydroxyapatite, releasing calcium and phosphate ions into the surrounding solution
Ca10(PO4)6(OH)2+H+→Ca2++HPO4 2- +H2O
What causes pH to drop below 5.5? When?
Lactic acid; when buffering effects of saliva are ineffective
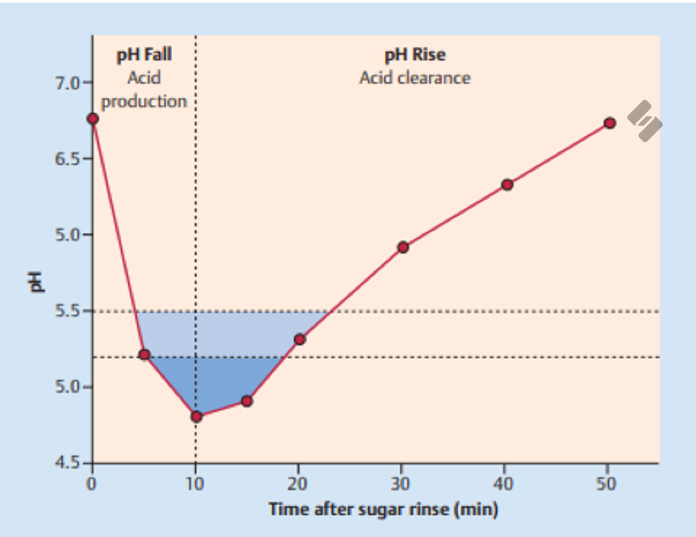
What is this called?
The Stephen Curve
What does The Stephen Curve represent?
The amount of time it takes for pH to fall below the critical level and rise back to normal levels
How long does it take for acid production to cause the pH to fall below 5.5?
5-10 min
How long does the pH typically stay below 5.5?
20-30 min
When should you brush your teeth when correlating with eating and why?
Immediately before you eat: with a fluoride toothpaste, this gives a slight protective layer to the tooth surface
1 hour after you eat: This allows for adequate time to pass to avoid the tooth surface being brushed into after being softened by the acids
What occurs at an oral plaque pH of 5.5?
Tooth enamel demineralization caused by an oral bacteria-induced increase in acidity
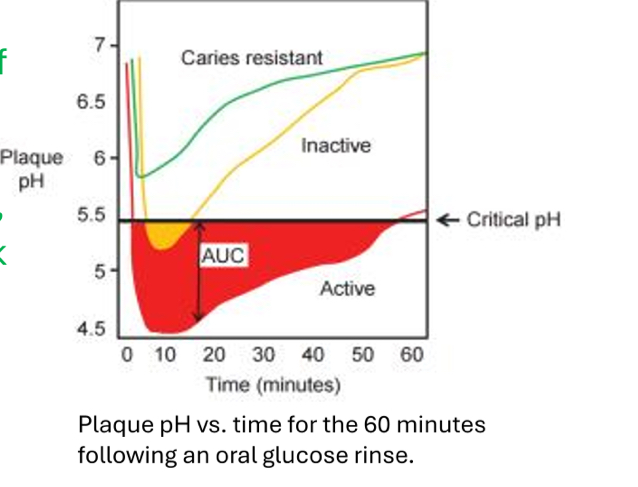
What does the green line represent?
A low-risk patient; someone who is caries-resistant
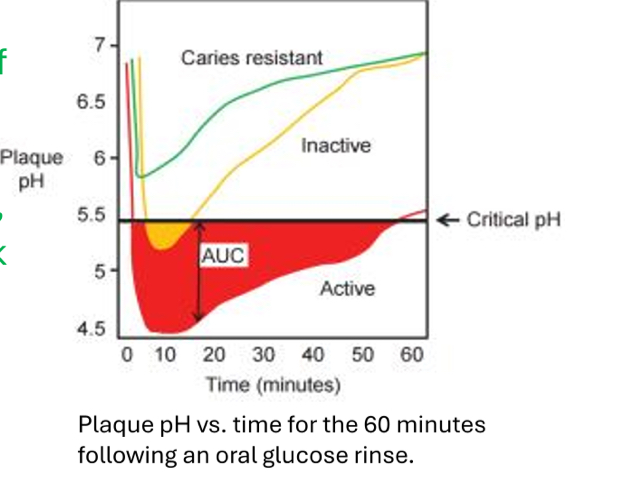
What does the yellow line represent?
A moderate risk patient
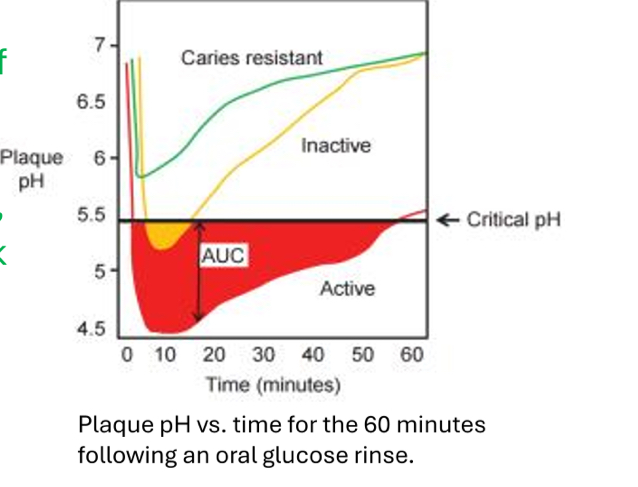
What does the red line represent?
A high risk patient, much longer time for return to baseline pH
Prevention measures from erosion caused by sugary and acidic beverages
Drink in moderation
Use a straw
Drink in one sitting or with food
Drink water to rehydrate
Wait an hour to brush after
Use fluoride
Drink milk, it is neutral pH and has a healthy amount of natural sugars