FUNCHEM 22: Importance of free energy in biochemical processes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What is entropy? and unit for entropy
Entropy is the measure of randomness
j/k mol
Why is entropy temperature dependent?
`the higher the temperature, the more energetic the molecules, the greater the randomness, increase in entropy
What happens to the entropy when a reaction produces more gas molecules
Increase in entropy
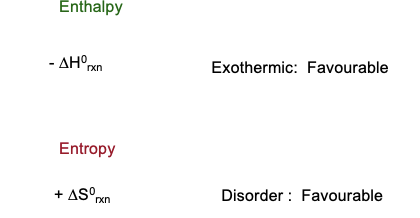
What is Gibbs Free Energy?
Gibbs Free Energy is the energy associated with a chemical reaction, that can be used to do work
How is H, T and S related?

If the change in G is negative, is the reaction spontaneous and what is the sign of H and S
The reaction is spontaneous and it is a negative enthalpy change and a positive entropy change
If the change in G is positive, is the reaction spontaneous what is the sign for H ad S
The reaction is non spontaneous and it is a positive enthalpy change and the entropy is negative