Unit 3 Cellular Energetics
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
What are Enzymes?
Enzymes are biological catalysts that speed up biochemical reactions
Enzymes are macromolecules
Most enzymes are PROTEINS
Enzyme structure is very specific resulting in each enzyme only facilitating one type of reaction
Tertiary shape must be maintained for functionality
Have a region called an active site
What does the active site interact with?
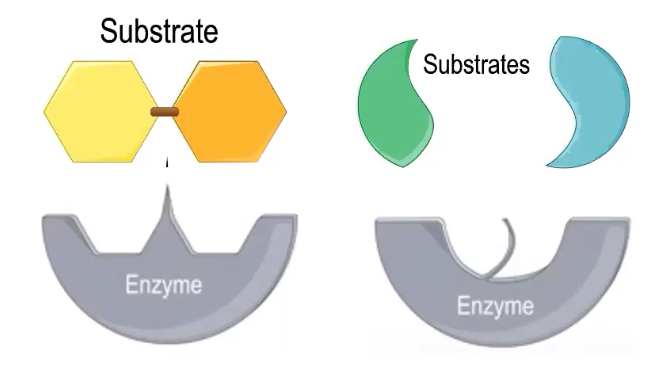
A molecule that can interact with an enzyme (active site) is the substrate
Enzymes have an active site, which SPECIFICALLY interacts with the substrate
Has a unique shape and size
Can have chemical charge(s) or not
Physical and chemical properties of the substrate MUST be compatible
SLIGHT changes can occur to align with substrate
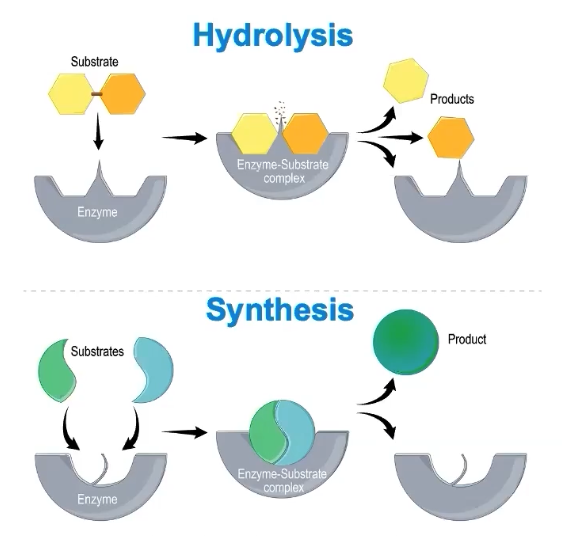
How do enzymes function in synthesis or digestion reactions/hydrolysis?
Enzyme names often indicate the substrate or chemical reaction involved
Enzyme names often end in -ase
Ex. Sucrase is an enzyme that digests sucrose
Enzymes are reusable
Not chemically changed by reaction
Cells typically maintain a SPECIFIC ENZYME concentration
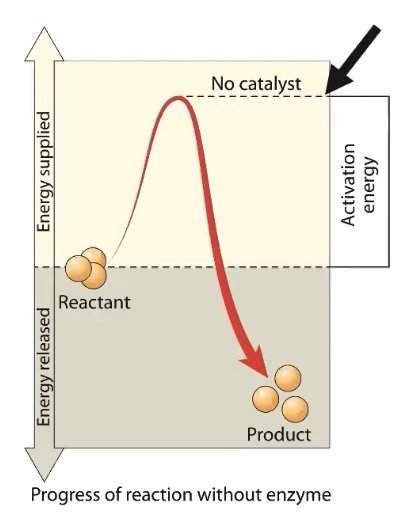
How do enzymes affect the rate of biological reactions?
All biochemical reactions require initial starting energy, called activation energy
Some reactions result in a net RELEASE of energy, and some reactions result in a net ABSORPTION of energy
Typically, reactions resulting in a net release of energy REQUIRE LESS ACTIVATION energy compared to reactions resulting in net absorption of energy
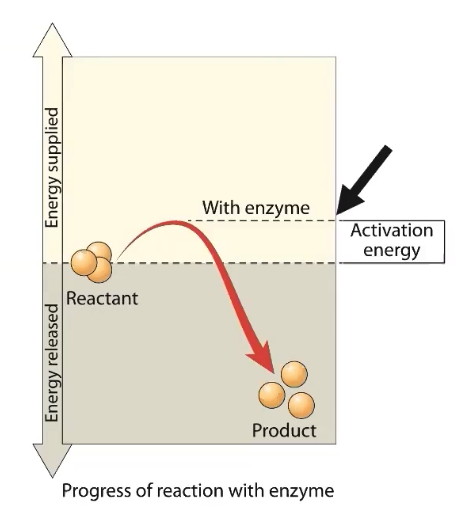
Enzymes LOWER the activation energy requirement of all enzyme-mediated reactions, accelerating the rate of reactions
What is a controlled experimentand what are the two types?
A scientific investigation
Two types of tests set up in a controlled experiment
Control test (group)
Generates data under conditions with no treatment/no manipulation
Generates data under normal/unchanged conditions
Considered baseline data
Experimental test (group)
Generates data under abnormal/unknown conditions
Generates data under treated/manipulated conditions
Test results are often compared with control test results to help determine possible impacts of a treatment/manipulation
What is a controlled group used for?
Used for comparison
Negative Control
Not exposed to the experimental treatment or any treatment known to have an effect
Positive Control
Exposed to a treatment that has a known effect
Not exposed to the experimental treatment
Both types of controls can be used to validate experimental procedures
*Controlled variables are aspects of an experiment that could be changed but are intentionally not changed
What changes to the molecular structure of an enzyme result in denaturation?
Changes in the conformational shape of the enzyme
Changes in environmental temperature
Changes in environmental pH
Enzyme denaturation is TYPICALLY IRREVERSIBLE, and the catalytic ability of the enzyme is lost or severely decreased
HOWEVER, in some cases, enzyme denaturation is reversible, regaining the catalytic ability
What are the effects of enzyme activity efficiency from environmental temperature?
Optimum temperatures
Range in which enzyme-mediated reactions occur the fastest
Reaction rates change when the optimum temperatures aren’t maintained
Environmental increase in temperature
Initially increases reaction rate
Increased speed of molecular movement
Increased frequency of enzyme-substrate collisions
Temperature increases outside of the optimum range result in enzyme denaturation
Environmental decrease in temperature
Generally slows down the reaction rate
Decreased frequency of enzyme-substrate collisions
Does not disrupt enzyme structure, no denaturation
What are the effects of enzyme activity efficiency from environmental pH?
pH measures the concentration of hydrogen ions in solution
Measured on a logarithmic scale
Small changes in pH values equate to large shifts in hydrogen ion concentration
Ex. pH 6 has 1ox more hydrogen ions in solution compared to pH 7
Optimum pH
Range in which enzyme-mediated reactions occur the fastest
Changing the pH outside of this range will slow or stop enzyme activity
Enzyme denaturation can occur as a result of increases and decreases outside of the optimum range
Changes in hydrogen ion concentration can disrupt hydrogen bond interactions that help maintain enzyme structure
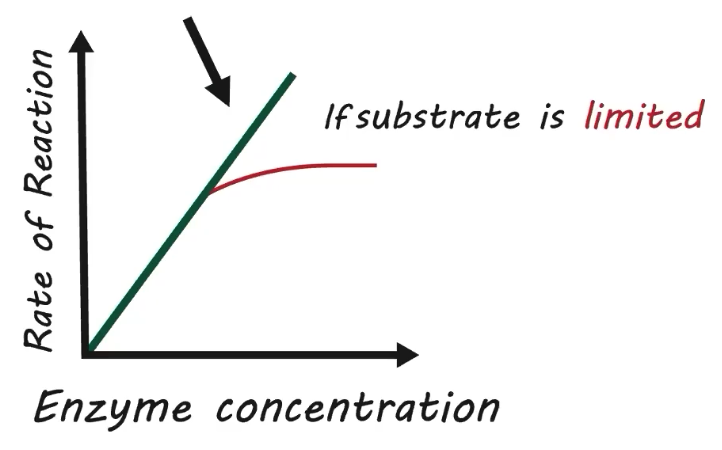
How does concentrations of substrates and products affect the reaction rate
Initial increases in substrate concentration increase reaction rate
More substrates mean more opportunity to collide with the enzyme
Substrate saturation will eventually occur
Results in no further increase in rate
Reaction rate will remain constant if saturation levels are maintained
Increased concentration of products decreases the opportunity for the addition of substrate
Matter takes up space
More products in an area means a lower chance of enzyme-substrate collisions
Slows reaction rate
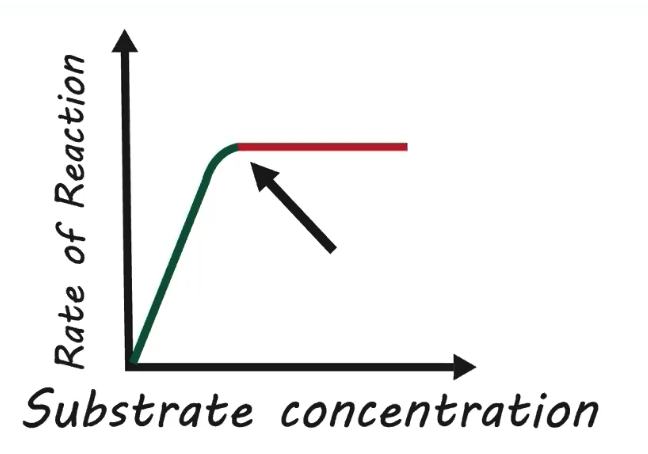
How does enzyme concentration impact reaction rate?
Less enzyme = slower reaction rate
Less opportunity for substrates to collide with active sites
More enzyme = faster reaction rate
More opportunity for substrates to collide with active sites
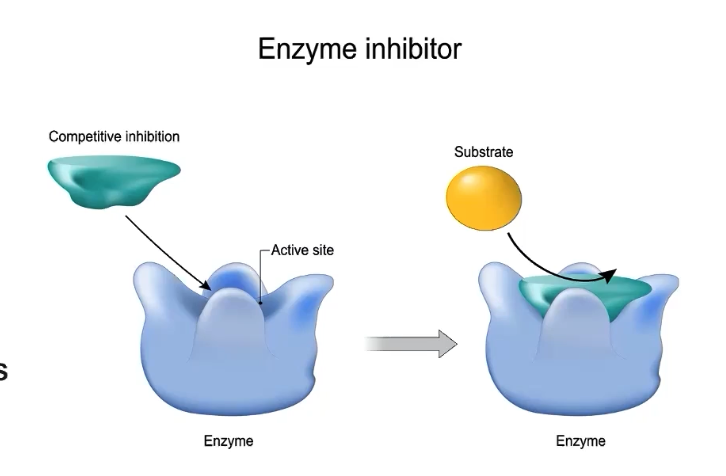
What are competitive inhibitors?
Molecules that can bind reversibly or irreversibly to the active site of the enzyme
COMPETES with the normal substrate for the enzyme’s active site
If inhibitor concentrations exceed substrate concentrations, reactions are slowed
If inhibitor concentrations are considerably lower than substrate concentrations, reactions can proceed normally
If inhibitor binding is irreversible, enzyme function will be prevented
If the inhibitor binds reversibly, the enzyme can regain function once the inhibitor detaches from the active site
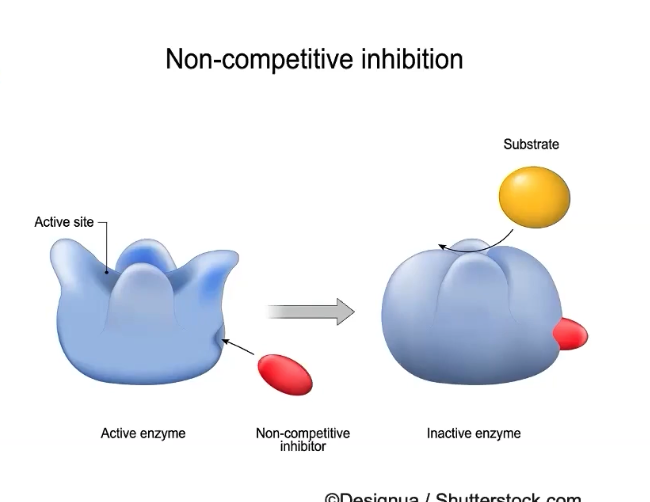
What are no competitive inhibitors?
Enzymes can have regions other than the active site to which molecules can bind, called an allosteric site
Noncompetitive inhibitors
DO NOT bind to the active site
BINDS to the allosteric site
Binding causes conformational shape change
Binding prevents enzyme function because the active site is NO LONGER available
Reaction rate decreases
Increasing the substrate cannot prevent the effects of noncompetitive inhibitor binding
Why do all living system require constant input of energy
Sunlight is the main energy input for living systems
Autotrophs capture energy from physical sources, like sunlight, or chemical sources, and transform that energy into energy sources, usable by all cells.
During every energy transformation process, some energy is unusable, often lost as heat
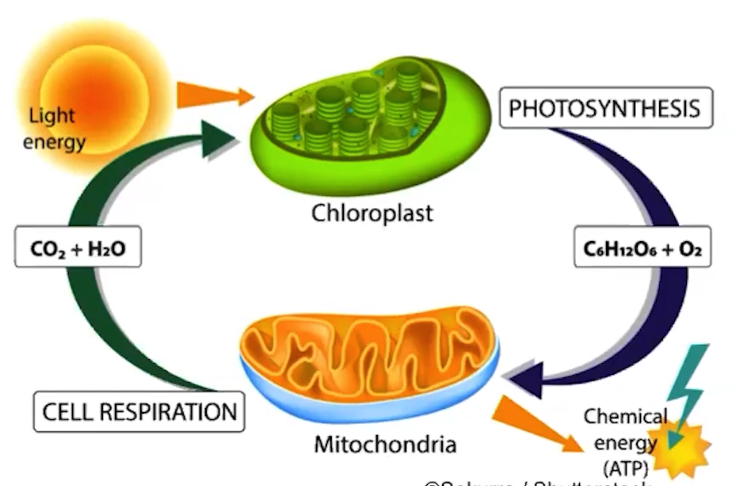
Life requires a highly ordered system and does not violate the second law of thermodynamics
Every energy transfer increases the disorder of the universe
Living cells are not at equilibrium; there is a constant flow of materials in and out of the cell
Cells manage energy resources by energy coupling. Energy-releasing processes drive energy-storing processes,
How are pathways in the biological system sequential?
Within a chemical pathway, the product of one reaction can serve as a reactant in a subsequent reaction.
The sequential reaction allows for a more controlled and efficient transfer of energy
What is photosynthesis?
The biological process that captures energy from the Sun and produces sugars
Evidence supports the claim that prokaryotic photosynthesis by organisms, such as cyanobacteria, was responsible for the production of oxygen in the atmosphere
Photosynthetic pathways are the foundation of eukaryotic photosynthesis
Light-dependent reactions of photosynthesis in eukaryotes involve a series of pathways
Light-dependent reactions capture light energy by using light-absorbing molecules called pigments
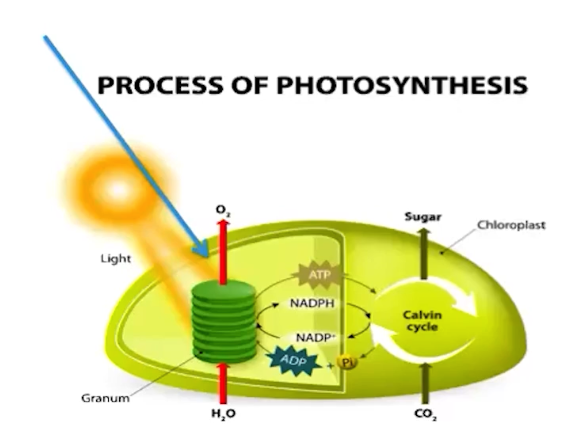
Pigments help transform light energy into chemical energy
Chemical energy is temporarily stored in the chemical bonds of carrier molecules, called NADPH.
Light-dependent reactions help facilitate ATP synthesis
ATP and NADPH transfer stored chemical energy to power the production of organic molecules in another pathway called the Calvin cycle
Oxygen is produced as a result of water hydrolysis
What is the role of chlorophyll in photosynthesis?
Capture energy from sunlight and convert it to high-energy electrons
What happens to chlorophyll electrons when light absorption occurs, and what is the importance of this?
Electrons will be energized. The energy from the electrons will be used to establish a proton gradient and reduce NADP+ to NADPH
What is a photosystem?
A photosystem is a light-capturing unit in a chloroplast’s thylakoid membrane.
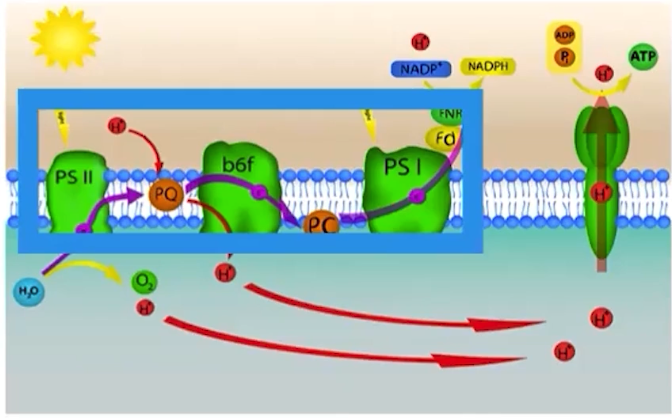
Why is the hydrolysis of water necessary as it relates to PSII and the light-dependent reactions?
The hydrogen molecules from the splitting of water are released into the thylakoid space and used to create an electrochemical/proton gradient
How are PSII and PSI functionally related to the electron transport chain (ETC)?
They pass as high-energy electrons to the ETC.
What is an electrochemical/proton gradient?
It is a difference in concentration of protons (Hydrogen ions) across a membrane.
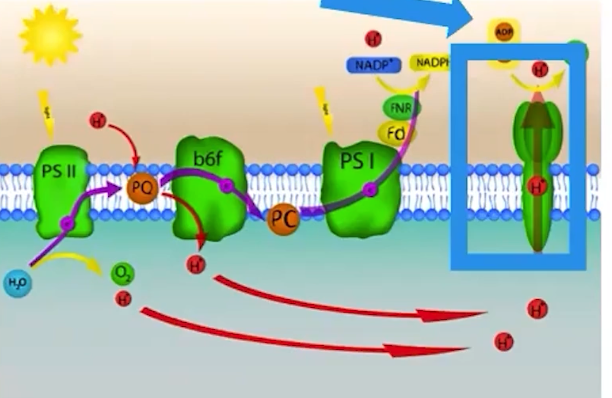
What is ATP synthase?
ATP synthase is an enzyme that creates ATP when protons pass through the enzyme
*Photosynthesis uses a form of passive transport to generate ATP from ADP
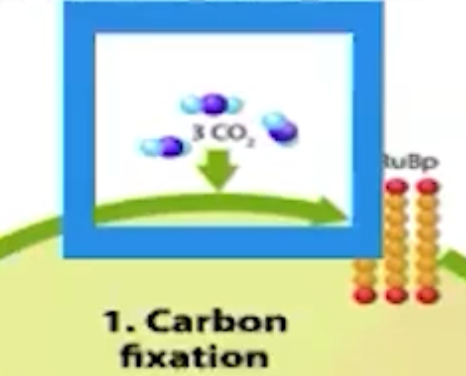
What does the Calvin cycle use?
ATP, NADPH, CO2, and produces carbohydrates
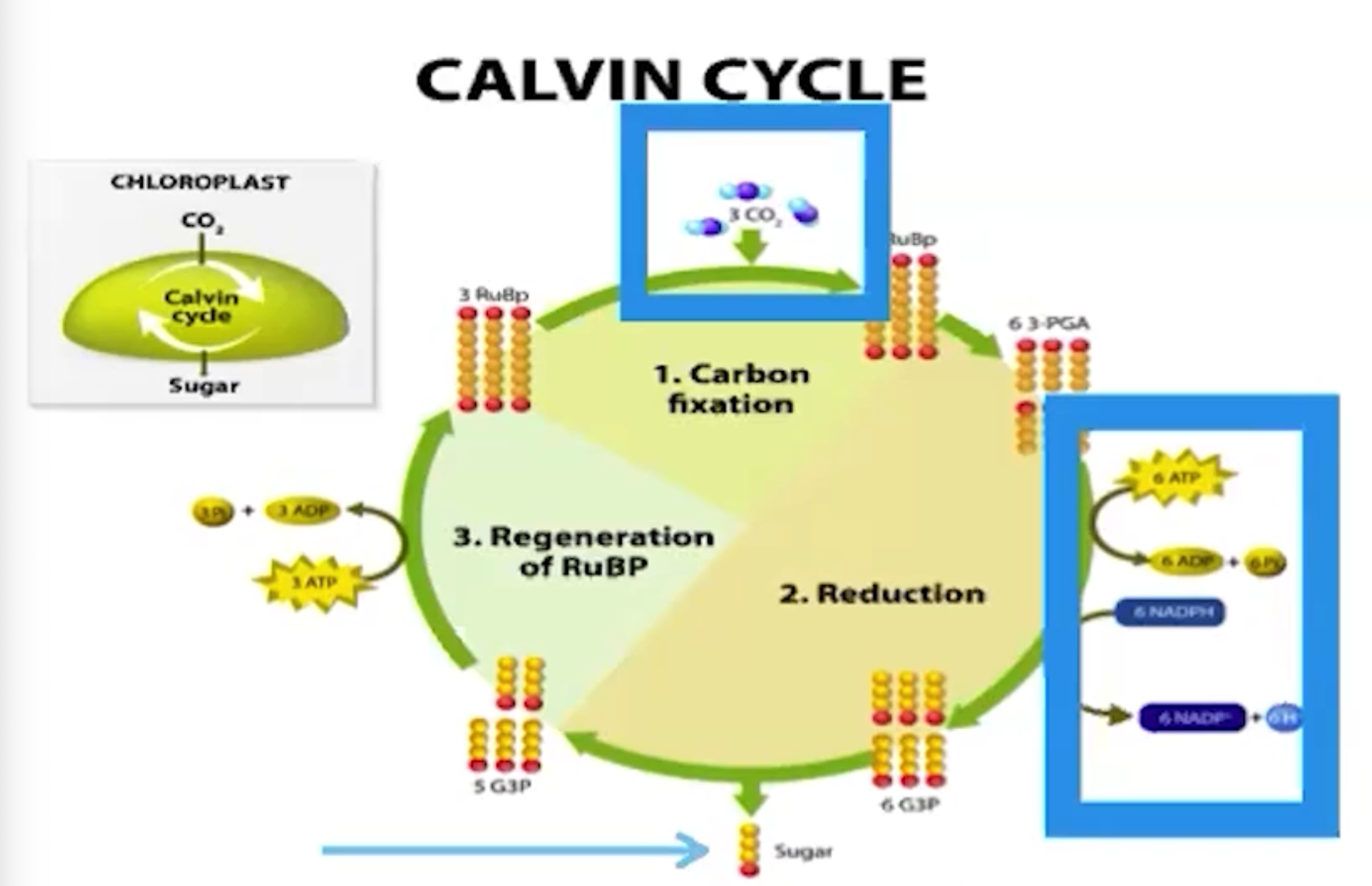
What is the ultimate goal of the Calvin cycle reactions?
Make organic products that plants need using the products from light reactions of photosynthesis

Where do plants and other organisms mainly get their carbon dioxide from?
Plants and other organisms mainly get their carbon dioxide from the environment
Fermentation and cellular respiration are processes that allow organisms to use energy stored in biological macromolecules
Cellular respiration and fermentation are characteristics of all forms of life
Releasing chemical energy from organic molecules, like glucose
OXYGEN IS NOT USED during the process of FERMENTATION but is USED during the process of CELLULAR RESPIRATION
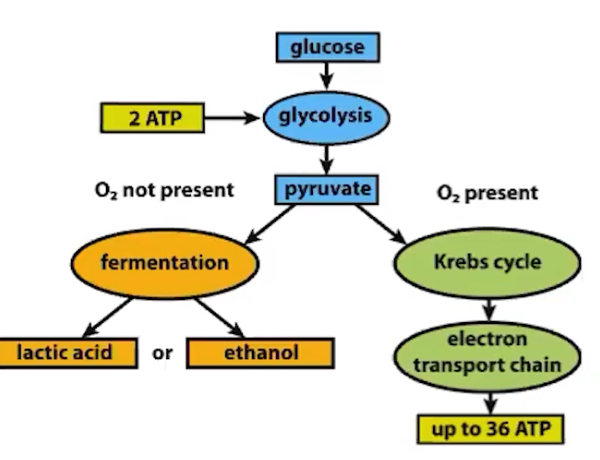
Fermentation and anaerobic respiration are not the same.
Products of fermentation are lactic acid or ethanol
Anaerobic respiration still has pyruvate
Cellular respiration in eukaryotes involves a series of coordinated enzyme-catalyzed reactions that capture energy from biological macromolecules.
Cellular respiration involves releasing chemical energy through the breakdown of glucose and creating an energy-storing molecule called ATP
ATP is used by all cells to do biological work
Cellular respiration involves multiple metabolic pathways:
Glycolysis— occurs in the cytoplasm
Pyruvate oxidation— occurs in mitochondria
Krebs (Citric Acid Cycle)— occurs in mitochondria
Electron transport— occurs in mitochondria
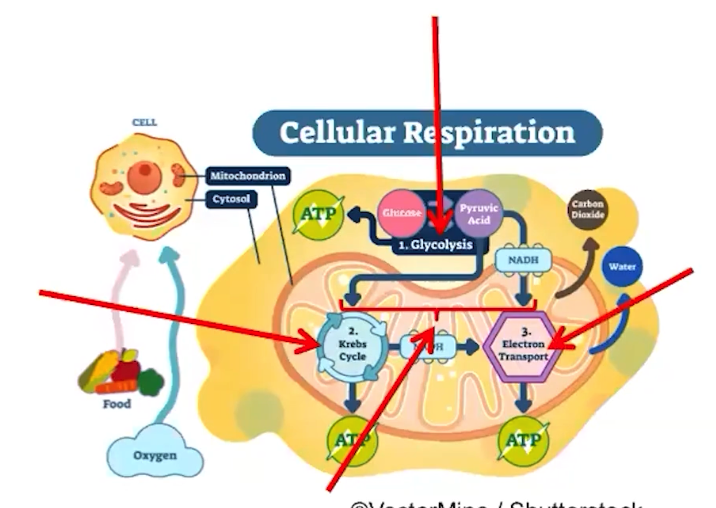
The electron transport chain transfers energy from electrons in a series of coupled reactions
Electron transport chain reactions occur in the membranes of chloroplasts and mitochondria, and in the cell membranes of prokaryotes
An ETC facilitates a series of coupled reactions used during cellular respiration
Electron transport chains allows for a more controlled and efficient transfer of energy
ETC uses electron energy to establish electrochemical/proton gradients across membranes

Electrons are delivered by electron carriers, called NADH and FADH2 to the ETC
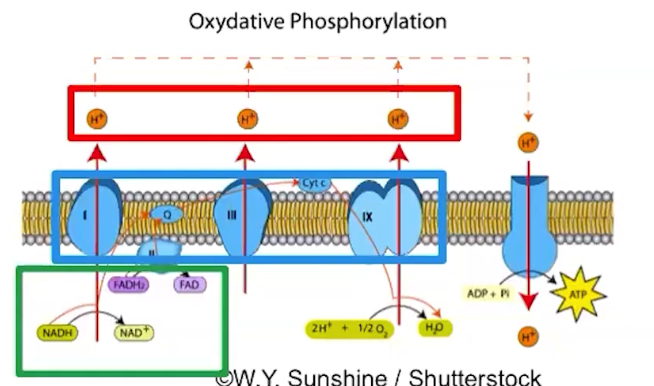
ATP synthase uses the proton gradient to synthesize ATP
ETC reactions occur in chloroplasts, mitochondria, and in the plasma membranes of some cells
The highly complex organization of living cells and living systems relies on a constant input of energy
ETC reactions are conserved processes
In eukaryotic cells, ETCs are located in the inner mitochondrial membrane and the internal membrane of the chloroplast
In prokaryotic cells, EETCs are located in the plasma membrane
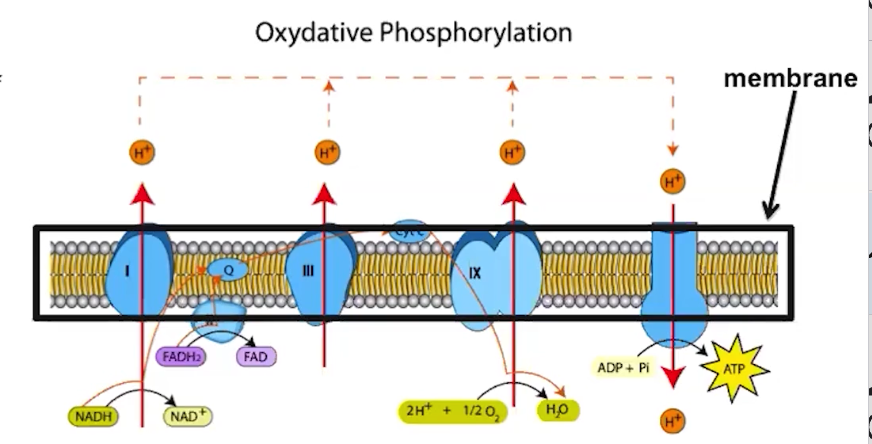
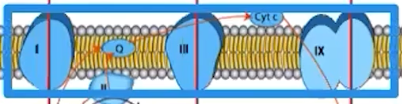
What does the ETC do?
Membrane proteins make up the ETC
ETC proteins facilitate a series of coupled reactions using the energy from electrons
High-energy electrons are donated by electron carriers, NADH and DADH
Active transport of protons establishes a proton gradient across the membrane
Proton gradients are maintained as a result of biological membrane impermeability to charged molecules/ ions
The flow of protons by chemiosmosis through ATP synthase drives ATP synthesis
The process of making ATP using the stored energy of a proton gradient is referred to as oxidative phosphorylation
NADH and FADH2 lose high energy electrons to the ETC = oxidation
ATP synthase adds an inorganic phosphate to ADP, resulting in an ATP molecule = phosphorylation.
Protons moving along the gradient (diffusion), through ATP synthase, power ATP synthesis