C1.2 Cell Respiration
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
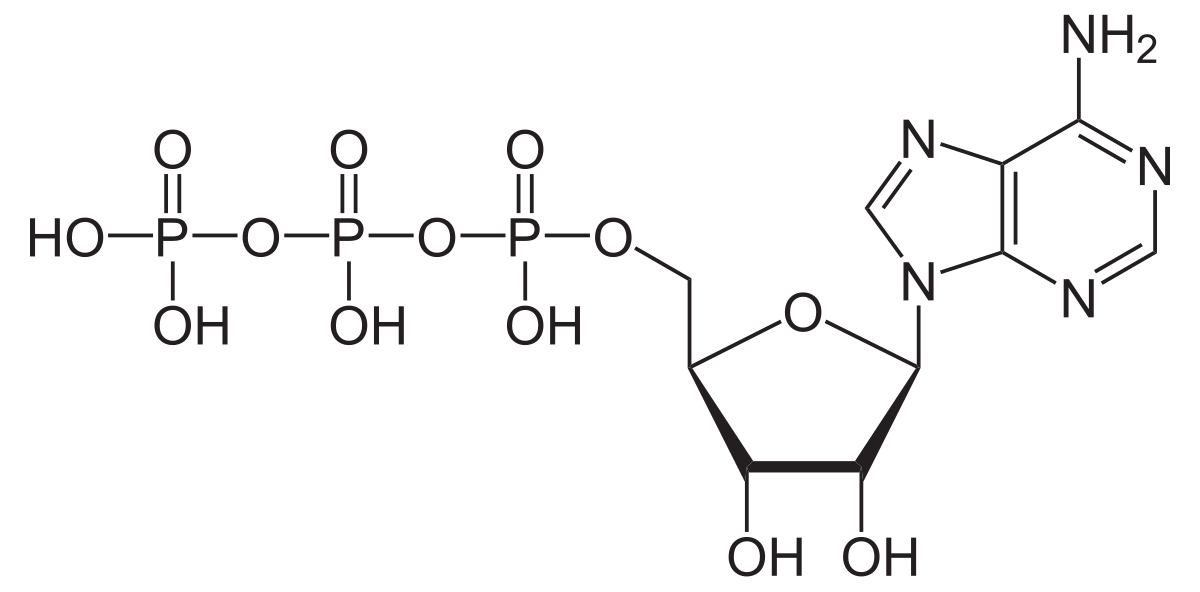
ATP structure
nucleotide with base adenine, pentose sugar ribose, 3 phosphate groups negatively charged and in a chain
properties of ATP that make it suitable for its role as the energy currency of the cell
water soluble - moves freely through aqueous solutions in the cell
stable in pH levels of cytoplasm and other close to neutral substances
cannot pass freely through membrane’s phospholipid bilayer - movement between membrane-bound organelles within cells can be controlled
ATP’s 3rd phosphate group is easily removed and reattached with hydrolysis and condensation reactions
hydrolyzing ATP to ADP and phosphate releases relatively small amount of energy, enough for many processes within the cell, without excess and conversion to heat
life processes within cells that ATP supplies with energy (3 main types of activity)
synthesizing macromolecules
anabolic reactions that link monomers together are endothermic and unlikely to occur without being coupled with the conversion of ATP to ADP
1 or more ATP molecules used every time monomer is linked to growing polymer
ex. synthesis of DNA during replication, RNA in transcription, protein translation
active transport - pumping of ions or other particles across a membrane against concentration gradient
energy required to cause reversible changes to conformation of pump protein, different conformations for allowing particles to enter and exit differed sides of the membrane
ATP used to cause change from more stable to less stable configuration, change to more stable does not require energy
movements (of components of cells)
ex. vesicles move to transport materials within cells
changing shape of a cell, changes of shape sometimes used for locomotion
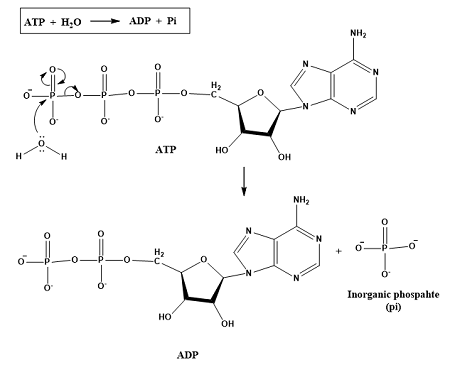
energy transfers during interconversions between ATP and ADP
energy released when ATP converts to ADP and PI because ATP contains more potential chemical energy than ADP due to its extra bond
energy required to convert ADP and phosphate back to ATP, can come from
cell respiration - energy released by oxidizing carbohydrates, fats, proteins
photosynthesis - light energy converted to chemical energy
chemosynthesis - energy released by oxidizing inorganic substances like sulfides
energy conversions between ADP and ATP are not 100% efficient, some energy is lost as heat
processes that require energy stop if all ATP within a cells is used up → cell degradation and death
prevented by continual regeneration of ATP from ADP and phosphate
cell respiration
function of life performed by all living cells, controlled release of energy from carbon (organic) compounds to produce ATP
respiratory substrates - mainly glucose and fatty acids
gas exchange
simple diffusion, oxygen enters cells and carbon dioxide exits cells simultaneously but independently, different process from cell respiration but interdependent
without gas exchange - lack of oxygen but excess of carbon dioxide
without cell respiration - gases cannot diffuse without concentration gradients produced by use of O2 and production of CO2
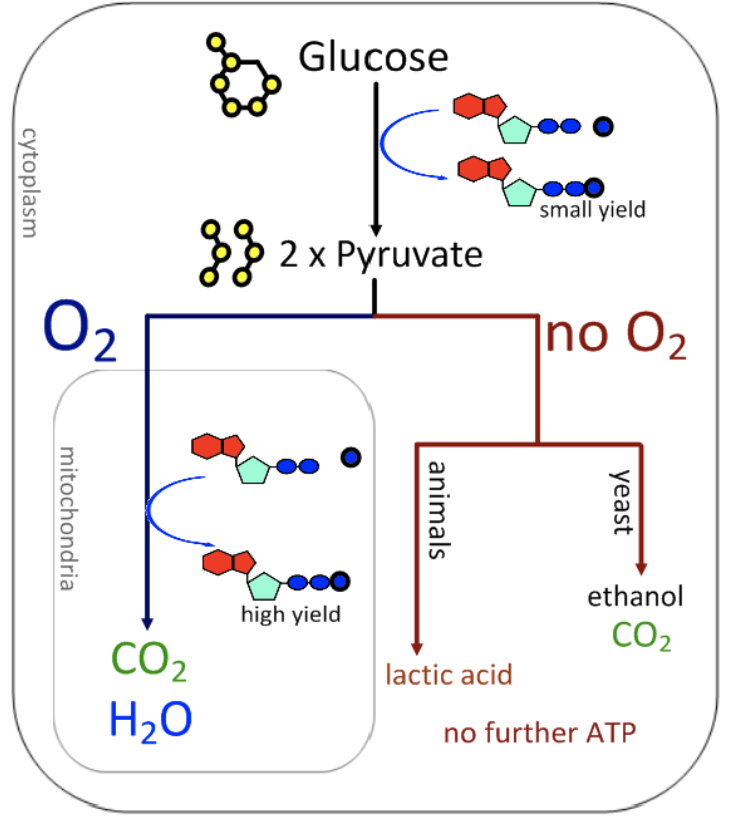
aerobic cell respiration
oxygen used as electron acceptor in oxidation reactions
can use carbohydrates, lipids, amino acids after deamination
waste products of carbon dioxide and water
much higher ATP yield - more than 30 ATP molecules per glucose
initial reactions in cytoplasm, more in mitochondria
anaerobic cell respiration
other substances used as electron acceptors in oxidation reactions
can only use carbohydrates
waste products of carbon dioxide and lactate or ethanol, water not produced
2 ATP per glucose
all reactions happen in cytoplasm, mitochondria not required
cellular respiration in humans
aerobic respiration - circulatory and respiratory system supply oxygen to most organs of the body rapidly enough for aerobic respiration
anaerobic respiration - can supply ATP rapidly over a short period of time, used when needed to maximize power of muscle contractions
lactate/lactic acid
waste product of anaerobic respiration in muscles
limit to concentration of lactate tolerated by human body - restricts how much anaerobic respiration can be done, short timescale for maximized power of muscle contractions
oxygen debt - demand for oxygen that builds up during period of anaerobic respiration, as oxygen is required to break down lactate
electron carriers
substances that can accept and lose electrons reversibly, often link oxidations and reductions in cells
oxidation - loss of e- from a substance
reduction - gain of e- from a substance
NAD (nicotinamide adenine dinucleotide)
main electron carrier in respiration
NAD+ + 2H+ + 2e- → NADH + H+
initially NAD+, substances oxidized in respiration by removing two H atoms, NAD+ accepts 2e- and 1 p+ from H atoms, becomes NADH + H+