Human Bio 2 Exam 1
1/113
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
Overview
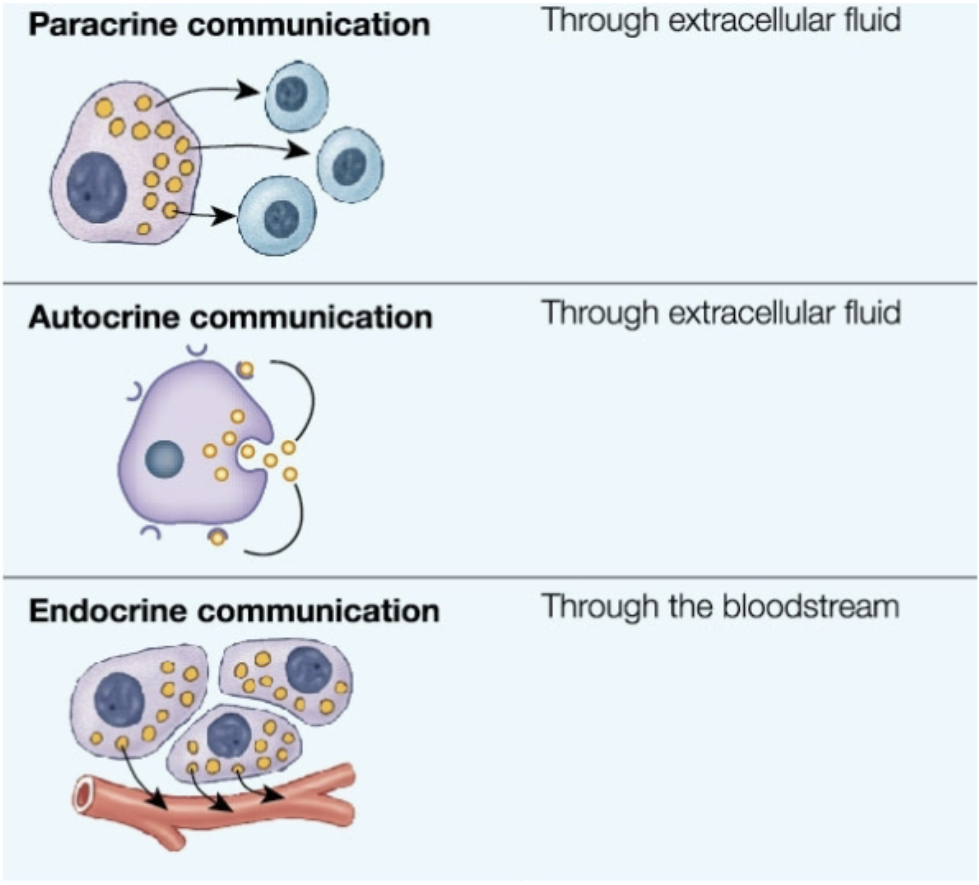
Chemical Signaling
Paracrine: cell → nearby cells via ECF
Chemical released from cell and has effects on nearby target cell
cell to cell, local signaling through extracellular fluid
ex. eicosanoids
Autocrine: cell → itself via ECF
chemical released from cell to effect itself
Endocrine: cell → distant cell via bloodstream
chemical produced by cell, hormone, released into bloodstream
effects distant target cell through interaction with target cell receptor
Intro to Endocrinology
Endocrine Glands:
ductless glands (specialized epithelium)
release chemical messengers (hormones) into blood stream
act on distant target cells
effects on target cells via interaction with receptors
Other organs beyond the classical endocrine and
neuroendocrine organs also produce hormones
primary endocrine organ:→ primary function to make and produce hormones
Hypothalamus
Pituitary gland
thyroid gland
adrenal glands
pineal gland
parathyroid glands
secondary endocrine organ → organ that has its own function but also has ability to make a produce hormones
heart
kidney
digestive system
adipose (fat)
gonads
Classification of Hormones: Structure
3 types: Amines, Lipid derivatives, peptides
Hormones = chemical messenger through bloodstream
1. Amines: amino acid derivatives
most derived from → tyrosine
thyroid hormone, epinephrine, norepinephrine, dopamine
melatonin (derived from triptifan)
2. Lipid Derivatives:
Steroid Hormones: derived from → cholesterol
i.e. testosterone, progesterone, estrogen, aldosterone, cortisol, corticosterone, Vitamin D
Eicosanoids: derived from → arachidonic acid (paracrines)
paracrine chemicals
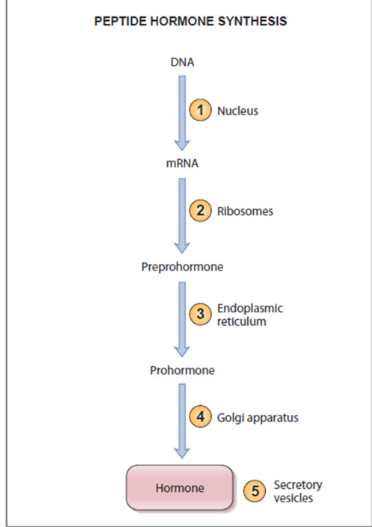
3. Peptide Hormones: 3-200 amino acids
majority of hormones
may be glycoproteins
divided into groups depending on size:
smaller amino acids chains → peptide hormones
larger amino acid chains → protein hormones
First synthesized as prohormones (inactive protein form)
undergo post-translational processing → activated
stored in secretory vesicles
released via exocytosis
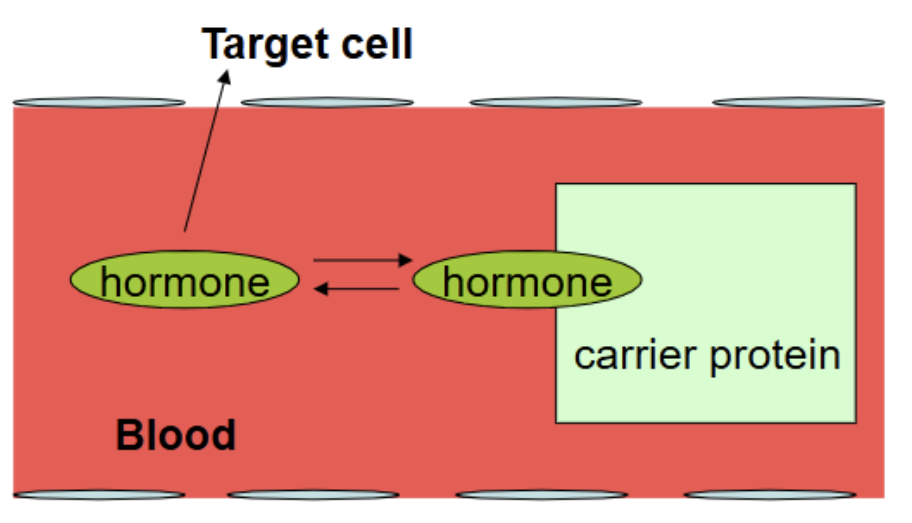
Hormones in the Blood
most hormones can travel through blood freeform (on its own) → eg peptides and amines
some hormones are hydrophobic (eg. lipid derived hormones) → travel through blood via carrier protein
Transport
hormones either circulate in blood either:
free form/unbound → most hormones
bound to carrier protein (some steroid hormones and some thyroid hormone)
Metabolic Clearance → how hormones are broke down
uptake by target cell and degradation
ideal → hormone interacts with target cell → cell degrades hormone
metabolic degradation: liver and kidney break down hormone → metabolites (break down products)
excretion of metabolites of hormone from blood:
urinary excretion
bile (feces)
very small amounts of hormone excreted in intact (unmodified form) → most degraded into metabolites
Bioavailability
amount of hormone available to bind and act upon target cell → ability to bind to target cell
Half-life
time needed for concentration of hormone in blood to decrease to 50% its initial concentration
Hormone Receptors
Hormone Receptors:
Cell must have appropriate receptor to be sensitive to hormone
most receptors have high specificity → sensitive to specific hormones
protein/glycoproteins
bind to hormones even through concentration in blood is very small (10-8 -10-12) → major effects based on receptor binding ability
Signal transduction: receptor binds to ligand → undergo conformational change → transduce signal to cellular response → signaling pathways
Types of receptors:
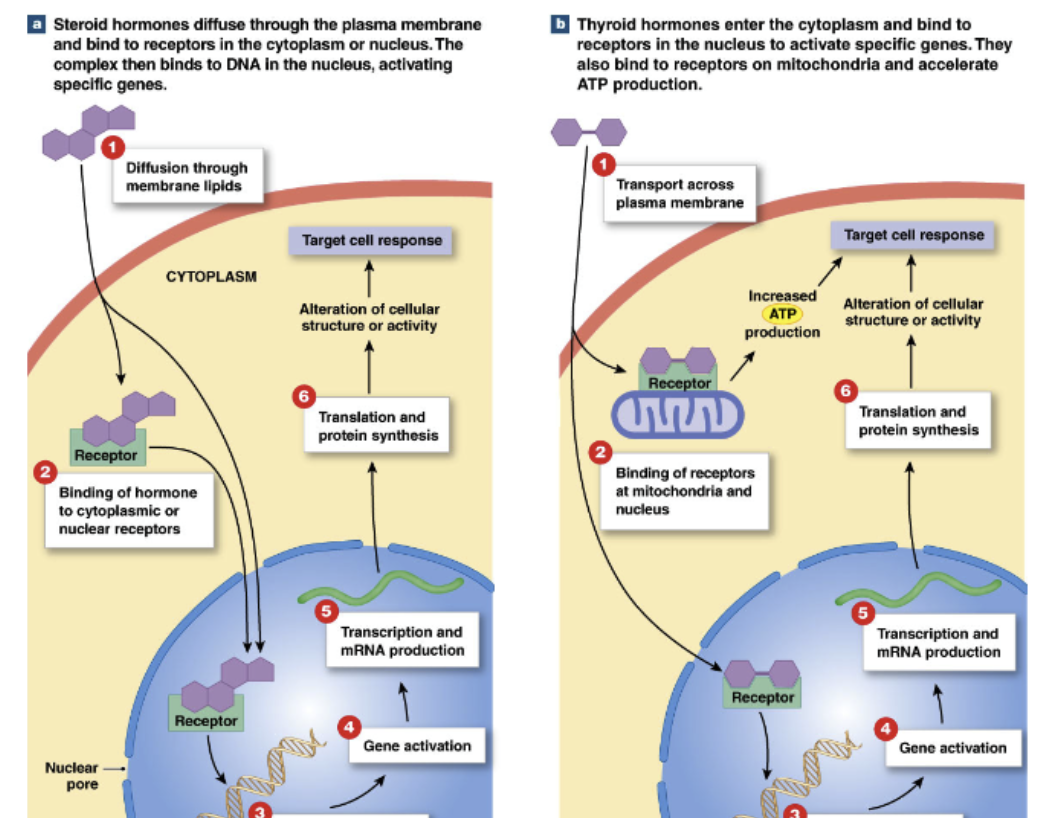
Intracellular receptors (in cell): steroid hormones, thyroid hormone
bind to hormones that can diffuse through plasma membrane (small, nonpolar) → steroid, thyroid hormone
effects: acts as transcription factor → alter gene expression
slow acting: long lag time to cellular response
slow response
Plasma Membrane Receptors: Peptide hormones, most amines
bind to hormones that can’t easily enter cell
amplification: second messenger systems
effects: alter activities of proteins in cell
fast acting: short lag time to cellular response
fast response
Intracellular Receptors
Steroid hormone receptors, thyroid hormone receptors
Steroid hormones
diffuse through plasma membrane →
bind to intracellular receptors in cytoplasm/nucleus →
form transcription factor →
moves to nucleus and binds to DNA →
activate specific genes → change gene expression of cell
Thyroid hormones
transported across plasma membrane into cytoplasm →
binds to intracellular receptor in nucleus → act as transcription factor
also bind to intracellular receptors on mitochondria → influence ATP production
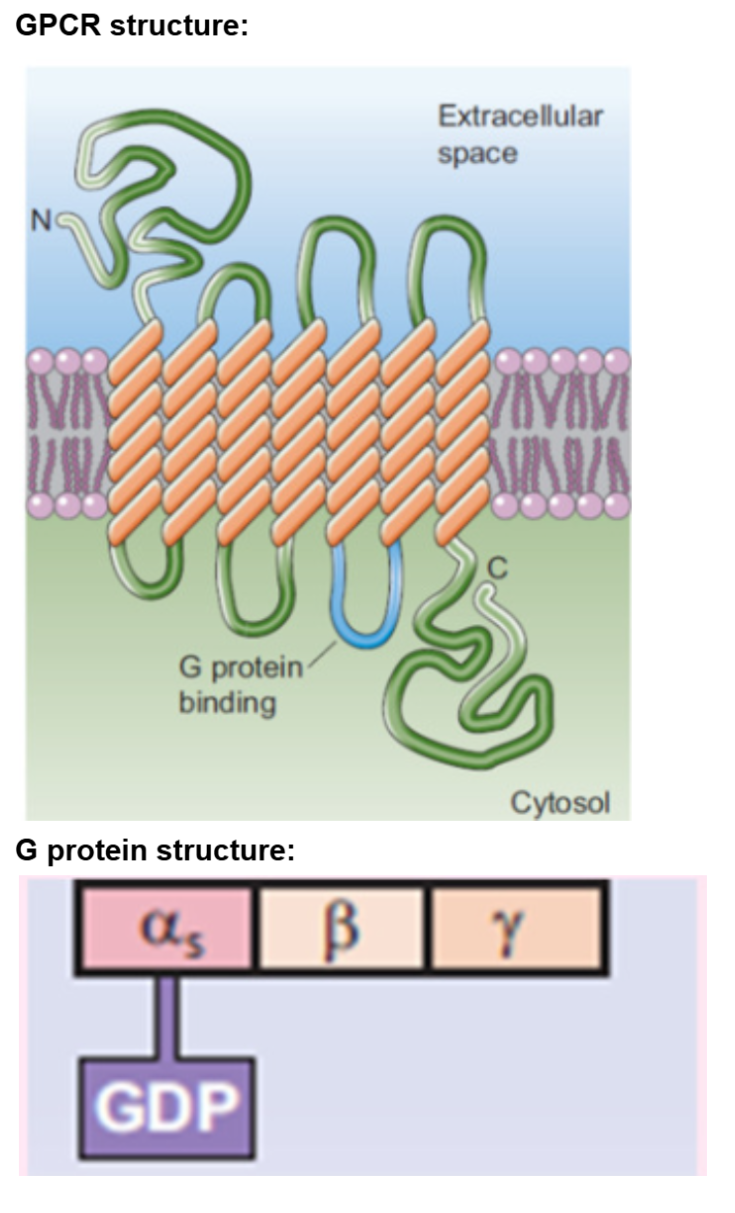
Plasma Membrane Receptors: GPCRs (metabotropic)
include: peptide hormones, most amine hormones
GPCR: G protein coupled receptors (metabotropic)
largest family of receptors
Basic Structure:
n terminal → extracellular side (amino group exposed)
c terminal → intracellular (carboxyl group exposed)
integral protein with 7 transmembrane domains (made of alpha helixes)
G protein interact with c terminal and intracellular loops of transmembrane domains
G proteins couple the hormone receptor to effector molecules within the cell
G Proteins:
interact with GTP or GDP and intracellular region of GPCR
polypeptide → quaternary structure (multiple proteins)
made of 3 protein subunits (heterotrimeric proteins):
alpha subunit: only subunit that interacts/binds to GDP or GTP
beta subunit
gamma subunit
Alpha subunit binds to guanosine diphosphate (GDP) or guanosine triphosphate (GTP)
GTP binding to alpha subunit activates G Protein
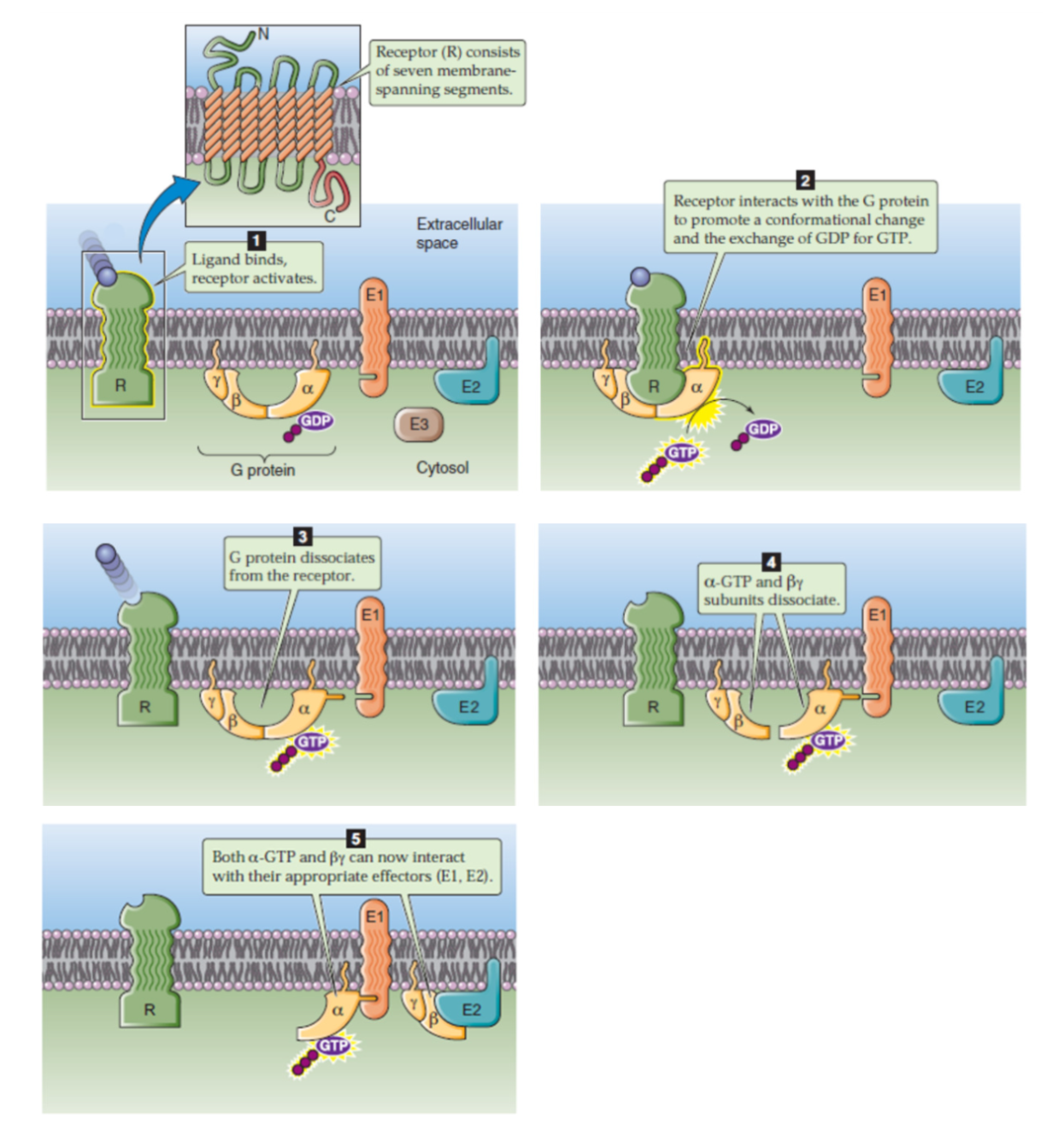
General Mechanism of GPCRs
ligand binds to receptor
G protein in inactive state
hormone binds to receptor → activates receptor → creates conformational change to structure of GPCR
G protein binds to receptor
receptor inieracts with G protein → conformational change and activate Alpha subunit (GDP→GTP)
activated receptor interacts with G protein
GDP replaced by GTP at alpha subunit of G protein → active form (with energy!!)
G protein dissociates from receptor
Activated Alpha subunit (with GTP) separates from beta-gamma dimer
Active Alpha subunit interacts with enzyme/effector molecule → utilize energy (GTP→GDP) → inactivated
dissociated subunits interact with effector molecules, eg enzyme, which now becomes activated/inhibited
Alpha subunit interacts with enzymes/effector molecules releasing energy via hydrolyzing GTP → GDP, becomes inactive again
beta gamma subunits can interact but usually doesn’t do much
Signal Amplification (dont memorize not necccessary to know all steps)
1 signal molecule → 1 million activated enzymes
protein phosphorylation (often but not always) important step
addition of phosphate group to protein by enzyme (kinase)
each step of transduction activates a bunch of molecules
signaling pathway:
reception
epinephrine bind to GPCR (activate 1 molecule)
transduction
each activated GPCR → activate 10² G protein
each g protein → activate 10² of enzyme
each enzyme → 10³ conversion
bunch of steps
response
activated enzyme → cleave millions of glucose molecules from glycogen
Receptor Regulation
Desensitization: Decreases a cell’s response to hormone
prolonged exposure to hormone → may lead to desensitization
decrease in # of receptors on plasma membrane
internalization of receptor:
receptor degraded in lysosomes or proteosomes
membrane holding receptors are pulled back into cell and degraded
downregulation of receptor number
making fewer receptors
Sensitization: Increases a cell’s response to hormone
may occur in response to low amounts of hormone
increase # of receptors on plasma membrane
stored receptors in vesicles fuse with membrane
upregulation: increase in number of receptors
hormones can regulate other receptor expression
ie estrogen regulates progesterone receptor expression
Phosphorylation of receptor
may lead to desensitization OR sensitization (depends on receptor and where its phosphorylated)
changing shape changes function
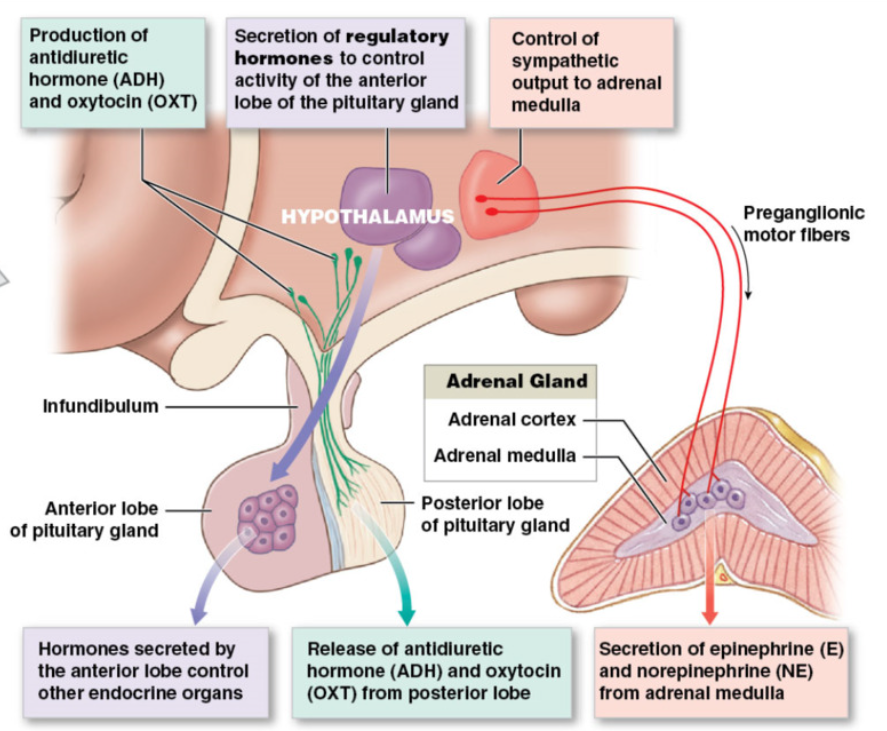
The Hypothalamus and Pituitary Gland
3 mechanisms of hypothalamus control over endocrine function
Anterior Pituitary: receive hormone → affect what hormone it releases
neuroendocrine cells: neurons that release hormones into circulation
release hormones in hypothalamus → travel bloodstream → influence hormone release from anterior pituitary
these hormones regulate hormonal production by other endocrine glands
anterior pituitary controlled by hypothalamic hormones (hypophyseal portal system)
Posterior Pituitary: synaptic terminals release hormone directly
neuroendocrine cells:
cell bodies in hypothalamus → axons run through infundibulum → synaptic terminals in posterior pituitary
hormones released from axon terminals in posterior pituitary → into general circulation
neuronal tissue
Adrenal medulla
release epinephrine and norepinephrine using SNS (already covered)
Posterior Pituitary
Release of 2 hormones: Oxitocin and Antidiuretic Hormone
Oxytocin: Peptide (9 amino acids)
pair bonding and maternal
Stimulus for release:
Breast → suckling of lactating breast
uterus → positive feedback mechanism with cervical stretch during labor
detected by sensory receptors in breast and uterus
Target Organs:
smooth muscle within breast (ducts of mammary glands)
smooth muscle of uterus
Effect: contraction of smooth muscle
milk let-down response
contraction of uterus
Antidiuretic Hormone (ADH) / Vasopressin: peptide (9 amino acids)
reduces amount of pee → affects kidneys → reabsorb water → dilute osmolarity
Stimulus for release
increased plasma osmolarity (hyperosmolarity >300 mOsm) → very sensitive
detected by osmoreceptors in hypothalamus
Target Organs
distal part of tubules in kidney
Effect: increased water reabsorption by distal part of kidney tubules → decrease osmolarity
ADH release stopped once osmolarity recovered
when ADH activated water pulled from urine when osmolarity is high, stopped when ADH is not released
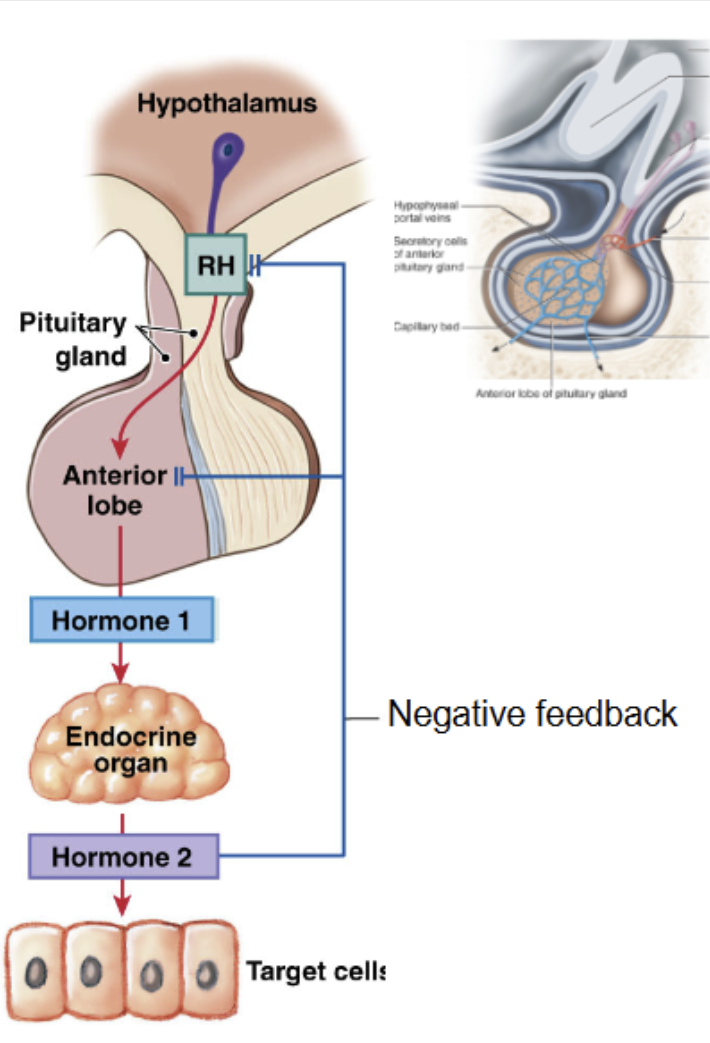
Hypothalamus hormones regulate Anterior Pituitary
GROWTH HORMONE release from anterior pituitary regulated by:
Growth Hormone Releasing Hormone (GHRH) → increase growth hormone
Growth Hormone Inhibiting Hormone (GHIH) (somatostatin (SS)) → decrease growth hormone
PROLACTIN release from anterior pituitary inhibited by PIH
Prolactin inhibiting hormone (PIH) → decrease prolactin
trigger: pregnancy, breast suckling (decrease PIH
target: mammary glands
effect: growth and development of mammary glands, milk synthesis, inhibit GnRH
THYROID STIMULATIMNG HORMONE (TSH) release regulated by:
Thyrotropin Releasing Hormone (TRH) → increase
ADRENOCORTICOTROPIC HORMONE (ACTH) release regulated by:
Corticotropin Releasing Hormone (CRH) → increase
LEUTINIZING HORMONE (LH) and FOLICLE STIMULATING HORMONE (FSH) regulated by:
Gonadotropin Releasing Hormone (GnRH) → increase
hormones regulated by hypothalamus
FLAPiG
GnRH→Fsh, LH
CRH→ increase ACTH
PIH → decrease prolactin
GHRH → increase GH
THYROID STIMULATING HORMONE release from anterior pit. regulated by
Thyrotropin Releasing Hormone (TRH)
Anterior Pituitary
5 types of secretory cells produces 6 peptide hormones
synthesis/release of anterior pituitary hormones → regulated by hormones from hypothalamus
hypothalamic hormones delivered to anterior pituitary via hypophyseal portal vessel
hypothalamic hormones affect cell that produce hormones:
GHRH/GHIH → Somatotrophs → growth hormone (GH)
PIH → Lactotrophs → prolactin (PRL)
TRH → Thyrotrophs → Thyroid Stimulating Hormone (TSH)
CRH → Corticotrophs → adrenocorticotropic hormone (ACTH)
GnRH → Gonadotrophs → follicle stimulating hormone (FSH) and Luteinizing hormone (LH)
Hypothalamic hormones influence → hormone producting cell in anterior pituitary → produce or inhibit hormone 2 → travel to target cells of endocrine organs → release hormone 3.
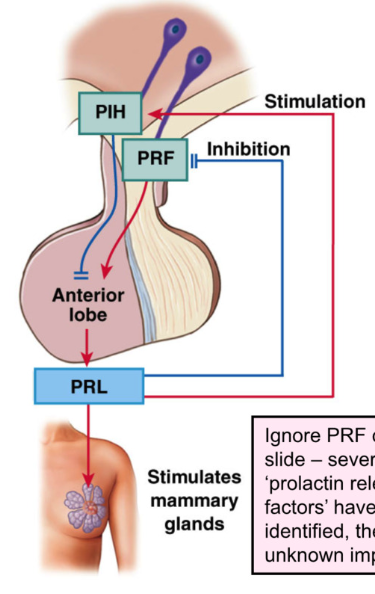
Anterior Pituitary: Prolactin
Prolactin: Peptide, tonic inhibitory control of PIH from hypothalamus
prolactin usually inhibited → when breast feeding → PIH decrease
PIH = Dopamine
prolactin release is stimulated by decreasing PIH (dopamine) release from hypothalamus
stimulus for release:
pregnancy and suckling on breast (decreases PIH release)
sensory receptors in nipples send afferent signal to hypothalamus
target organ:
mammary glands of breast
effect:
stimulate growth and development of mammary glands (levels rise during pregnancy
stimulate milk SYNTHESIS
Prolactin = feedback loop for GnRH → inhibits GnRH (gonadotropin releasing hormone) release from hypothalamus → inhibits FSH and LH
hyperprolactinemia → menstrual cycle irregularities in females, infertility, low libido in males
males can lactate with a bunch of prolactin
Anterior Pituitary: Growth Hormone
Growth hormone (aka somatotropin): peptide (needs both inhibitory and releasing hormone)
under stimulatory (GHRH) and inhibitory (GHIH) hypothalamic control
stimulus for release:
circadian rhythm → peak in release in early hours of sleep (varies with age)
target organs: most cells of body
GH effects on target hormones via 2 mechanisms:
1. Growth hormone binds to GH receptors directly on target cell (direct)
2. Major effect: Growth hormone stimulates production of insulin-like growth factors (IGFs/somatomedins) from liver → IGFs bind to receptor cells (indirect)
effect:
stimulates cell growth and division → increase protein synthesis
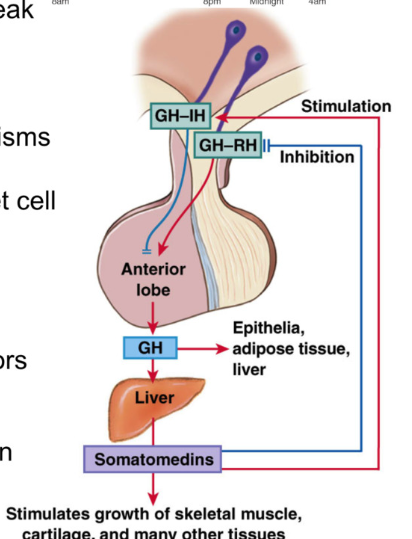
Effects of Growth Hormone
Liver: GH → production of IGFs, increased protein synthesis, increased synthesis of glucose
Muscle: GH → increased amino acid uptake and protein synthesis
adipose: stimulate lipolysis
visceral organs and glands: increased protein synthesis and cell proliferation
connective tissue/bone: increased amino acid uptake and protein synthesis, increase in linear growth by proliferation of chondrocytes and protein synthesis in cartilage
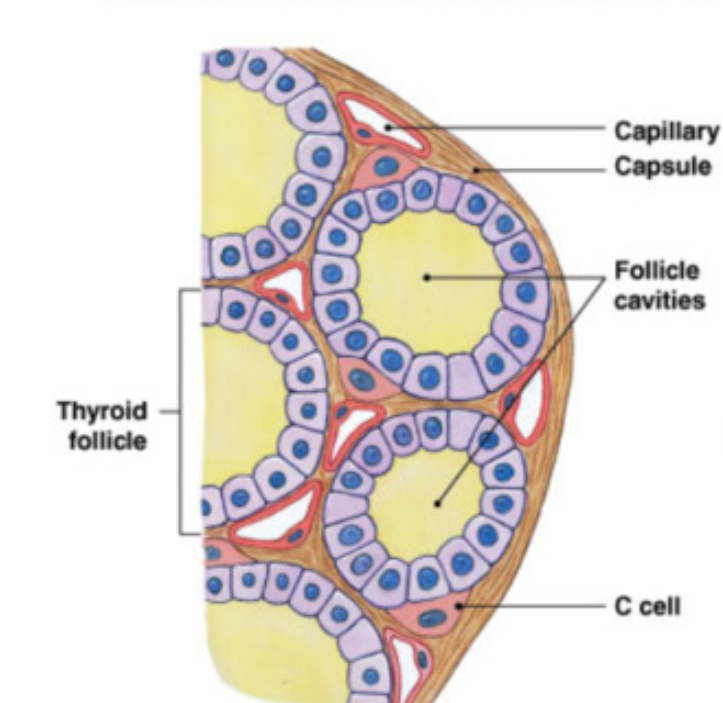
Thyroid Gland: anatomy
2 connected lobes just inferior to thyroid cartilage (highly vascularized)
follicle = smallest functional unit
fluid (colloid) filled sphere lined by simple cuboidal epithelial cells (follicle cells)
synthesis/release → thyroid hormone
Parafollicular cells: “C cells”
synthesis/release → calcitonin hormone
calcitonin released when there is too much Ca2+ in blood
inhibits osteoclasts
increase excretion of Ca²+ by kidney
prevent absorption of Ca2+ by digestive system
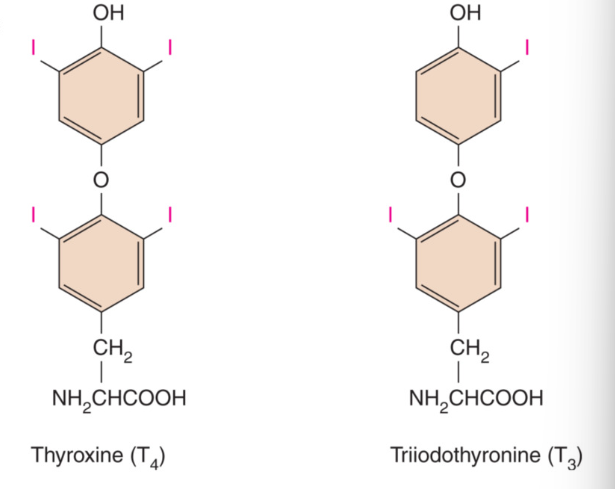
Thyroid Hormones: T4 and T3
amine
derived from 2 amino acid tyrosine
iodine = essential dietary element → required for synthesis of thyroid hormones
2 forms of thyroid hormones:
T4 : thyroxine
4 iodine atoms
most abundant form of thyroid hormone
T3 : Triiodothyronine
contains 3 iodine atoms
most biologically active form of thyroid hormone
T4 released into blood stream from thyroid gland can be diodinated into most active form T3 in some target cells including kidney and liver
Receptors→
cytoplasmic → storage
mitochondria → aTP
nucleus → gene transport
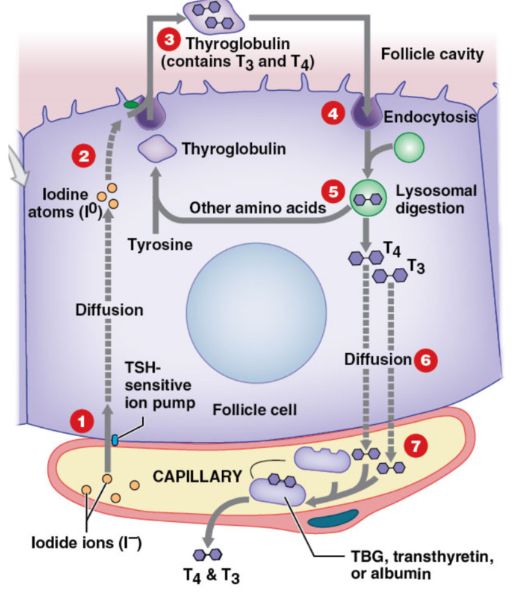
Synthesis of Thyroid Hormones
thyroglobulin = globular protein secreted by follicle into colloid → contains many tyrosine residues
Capillary beds transport iodide ions from blood → follicular cells in response to TSH (active transport)
Iodide ions converted → iodine atoms by thyroid peroxidase → combine iodine with thyroglobulin (protein)
transferred into colloid → T3 and T4 formed in thyroglobulin
endocytosis of thyroglobulin → back into follicular cell
lysosomes degrade thyroglobulin → release T3 and T4
thyroid hormones diffused out follicle cell → plasma
transported in plasma via carrier protein
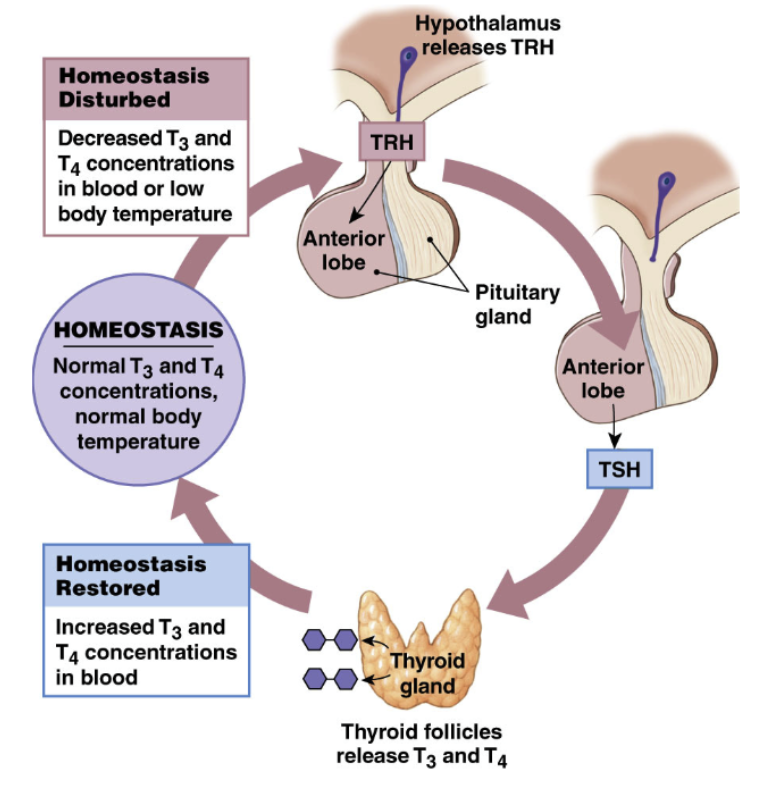
Regulation of Thyroid Hormone
Hypothalamus
TRH: Thyrotropin Releasing Hormone
Action: synthesis/release of TSH from anterior pituitary
Hypothalamus sends TSH → anterior pituitary via hypophyseal portal system → go to thyrotrophs → release TSH
Anterior Pituitary
TSH: Thyroid Stimulating Hormone
Action: Synthesis and release of thyroid hormones
TSH enter blood stream → transport iodide into thyroid gland
influence structure and growth of thyroid gland
Thyroid Gland
Thyroid Hormones
1. effects on target cells
2. negative feedback on hypothalamus and anterior pituitary
higher levels of thyroid hormone → stop TRH production by hypothalamus → stop TSH release by anterior pituitary → decrease thyroid hormone production
mediated by levels of thyroid hormone
Actions of Thyroid Hormones
Stimulates Growth and metabolism
Affect almost every cell in body
fast, strong, short increase in rate of cellular respiration → increase metabolism
Specific actions
increased metabolic rate (heat production) → increased body temp for children (little/no effect on adults)
increased HR and BP
stimulate red blood cell formation in kidney→ increase oxygen delivery
accelerate turnover of minerals in bone
affect osteoclasts and osteoblasts
3 receptor locations in cell (intracellular receptors)
Cytoplasmic receptors: storage
Mitochondria receptor: increase rate of ATP production
Nucleus: act as transcription factor → increase gene transcription
upregulation of NA/K pump, glycolytic enzymes
Pathophysiology: Hypothyroidism
deficient thyroid hormone
most common cause → iodine deficiency
symptoms of hypothyroidism (dont memorize)
tiredness weakness
dry skin
feel cold
hair loss
difficulty concentrating
constipation
weight gain and poor appetite
Hypothalamus release TRH → Anterior pituitary release TSH → but thyroid does not produce T4 and T3 because it does not have → produce more TRH and TSH
Pathophsyiology: Hyperthhyroidism
excess thyroid hormone
most common cause: grave’s disease (autoimmune disorder = body attacks itself)
antibody activates TSH
goiter and increased T4 and T3
production of thyroid stimulating antibody → mimics TSH → binds to TSH receptor → produce too much thyroid hormone → feedback loop tries to stop TSH → antibody says nah
ectopic antibody bound to thyroid
Parathyroid Glands
4 small glands embedded on posterior surface of thyroid
collection of parathyroid principle cells
secrete Parathyroid Hormone (PTH) in response to decreased blood Ca2+ levels
effects:
stimulates osteoclasts → eat bone → release Ca2+
enhances reabsorption of Ca2+ by kidney
stimulates formation of calcitriol (active vitamin D) by kidney → promotes absorption of Ca2+ from digestive system
action release PTH
trigger: low ca²+
effect: stimulate osteoclasts → increase renal Ca²+ resorption, increase calcitriol formation
calcium has multiple physiological roles (normal plasma levels → 8.8-10.2 mg/dL)
nerve and muscle excitation
muscle contraction
blood coagulation
bone mineral balance
intracellular signaling
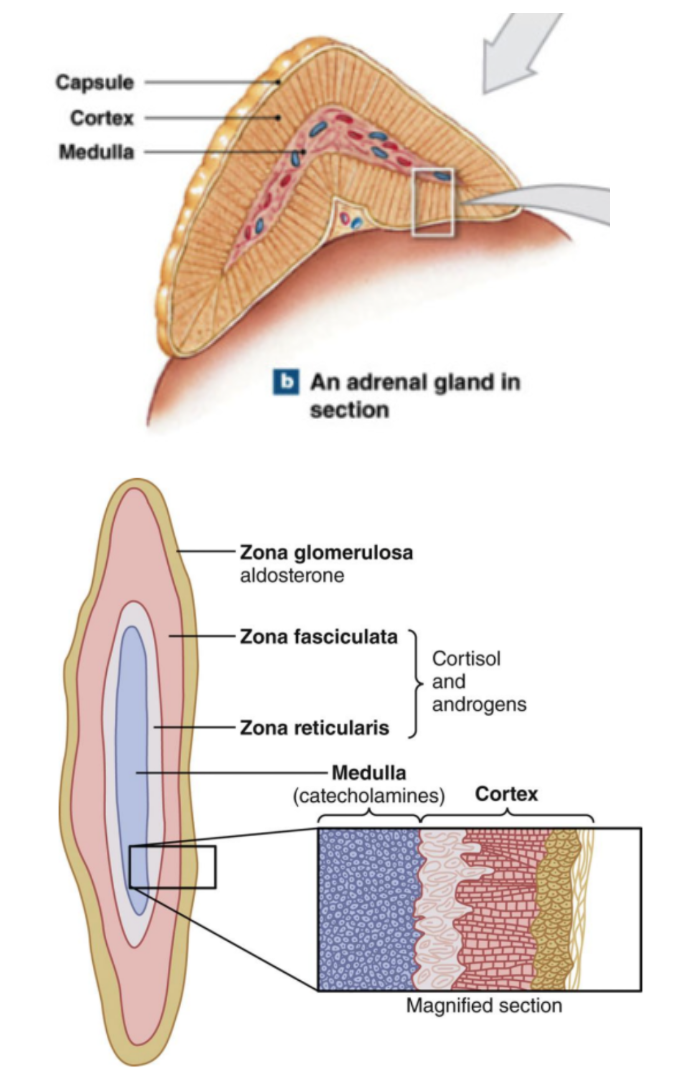
Anatomy of Adrenal Glands
Retroperitoneal (behind and above) above each kidney
composed of:
outer cortex → produce corticosteroids (2 dozen steroid hormones)
inner medulla → produce epinephrine and norepinephrine
outer cortex layers:
superficial
zona glomerulosa (release mineralcorticoids)
zona fasiculata (glucocorticoids)
zona reticularis (adrenal androgens)
deep
Hormones of Adrenal Cortex
All adrenocortical hormones are steroids → derived from cholesterol
Mineralocorticoids: Zona glomerulosa
regulate sodium and potassium levels in ECF
aldosterone: released when Na+ levels are low
reabsorption of Na+ and water from forming urine in kidney, sweat glands, salivary glands at expense of K+
Glucocorticoids: Zona fasciculata
Regulation of carbohydrate levels in ECF
anti-inflammatory properties
cortisol, corticosterone:
speed up rate of glucose synthesis (gluconeogenesis) and glycogen formation m
anti-inflammatory → reduces immune system function
Adrenal androgens: Zona reticularis
produce low levels of “weak” androgens → useful as precursors for production of estrogen and testosterone by other tissues
influence muscle mass and sex drive in adult women
Hypothalamic Pituitary Adrenal (HPA) Axis
Release of CRH (corticotropin releasing hormone) increased by stressors
hypothalamus produce CRH → enter hypophyseal portal system → effect corticotrophs in anterior pituitary → release ACTH (Adrenal Corticotropic Hormone) → travel through blood stream → adrenal gland → release cortisol
inhibition of release of CRH is initiated by cortisol (negative feedback loop)
cortisol → stop hypothalamus from CRH production → stop ACTH → stop cortisol production
chronic high levels of cortisol desensitize receptor cells in brain (hypothalamus)
effect: continued release of CRH → excess production of cortisol
Chronic stress → chronically high levels of cortisol
stress → CRH → ACTH → cortisol
gluconeogenesis
protein mobilization
fat mobilization
stabilize lysosomes
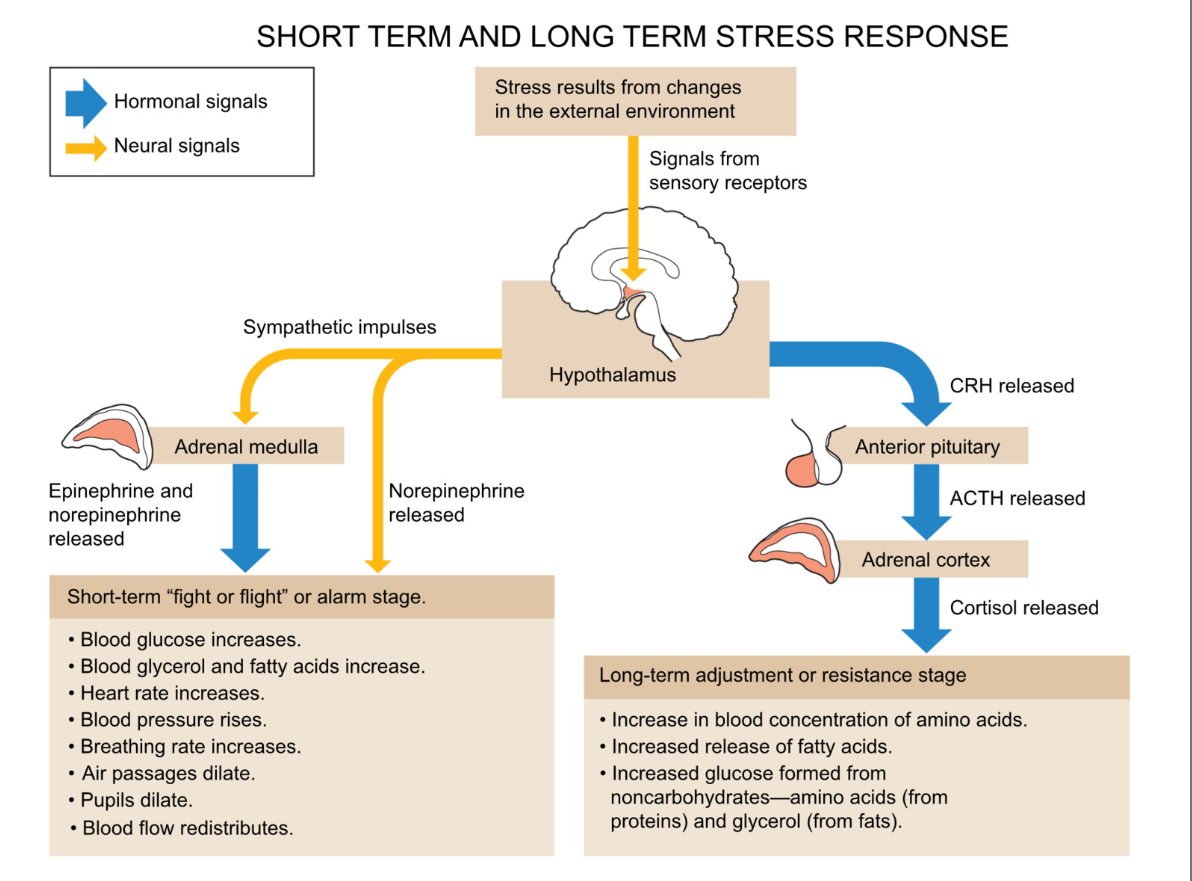
Short term and long term stress response
stress from external environment change
signals from sensory receptors → hypothalamus
→
short term stress (fight or flight/ alarm stage)
blood glucose increase
blood glycerol and fatty acids increase
HR and BP increase
air passage dilate
pupils dilate
blood flow redistribution
long term
increase in blood concentration of amino acids
increased release of fatty acids
increased glucose formed from noncarbohydrates → amino acids (from protein) and glycerol (from fats)
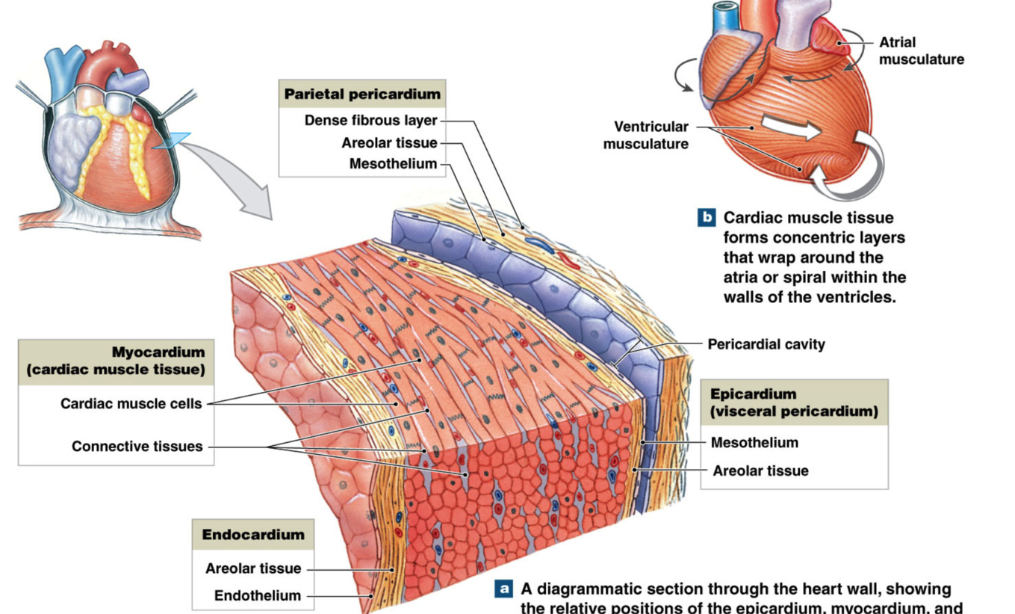
Heart Wall
superficial
parietal pericardium
outer membrane
pericardial cavity
epicardium (visceral pericardium)
myocardium
contain most cardiomyocytes
endocardium
inner lining of heart chambers
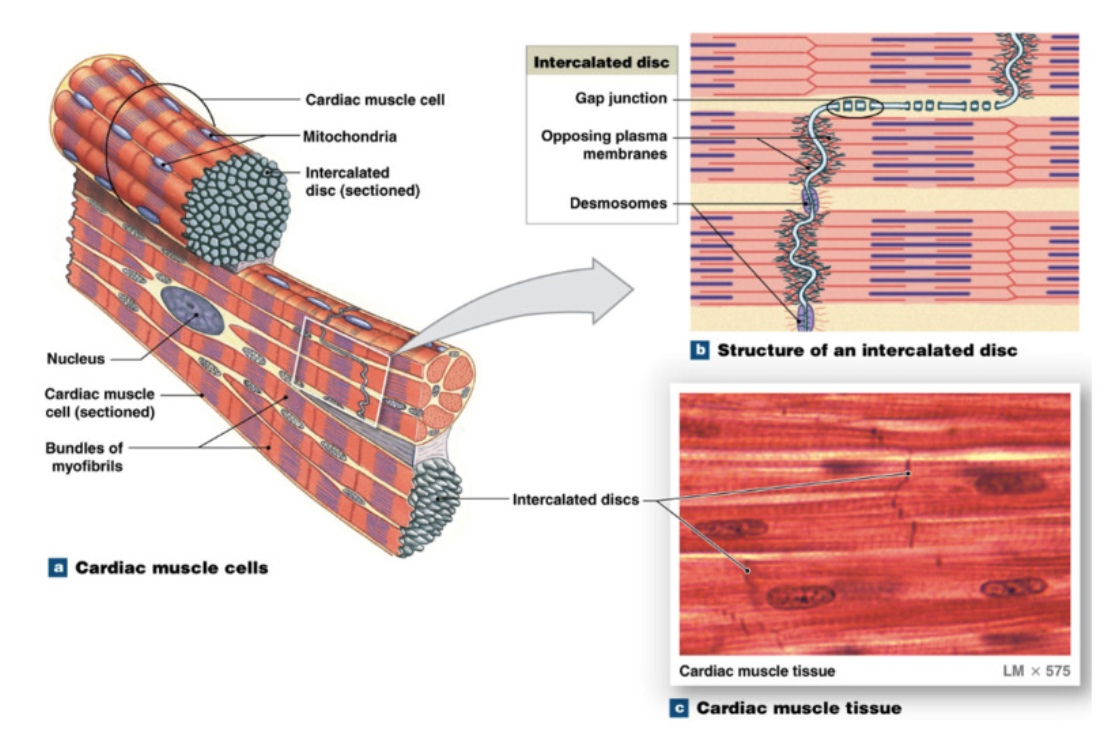
Cardiomyocyte Cells
cardiac muscle cells = cardiomyocytes
striated (orientation of sarcomeres)
50-100 um long, diameter 20 um (shorter and thinner than skeletal muscle cells)
branched at ends → contact other cardiomyocytes → create network of interconnected cardiomyocytes
mono nucleated (vs multinucleated skeletal muscle cells)
reduced sarcoplasmic reticulum system but extensive T tubule system
large and numerous mitochondria → lots of ATP use
Unique feature: Intercalated discs → contact point between cardiomyocytes
desmosomes: mechanical coupling → proteins holding cardiomyocytes together
gap junctions: electrical coupling (ESSENTIAL FOR HEART CONTRACTION) → protein channels allowing flow between cardiomyocytes
Major types of Cardiomyocytes
contractile cells → normal cardiomyocytes
bulk of atrial and ventricular tissue
contraction and transfer of electrical signals
conductive cells → specialized cardiomyocytes
generate and propagate its own electrical activity
communicate with other cells via connected pathway → conductile pathway
spread activity across contractile cells → induce contraction
skeletal vs cardiac muscle cells
in common:
sliding filaments → produce contraction
regulation of contraction via increase in intracellular calcium
calcium bind to troponin → move tropomyosin → free myosin binding site on actin
cardiac muscle only
influenced by autonomic nervous system
calcium from ECF AND SR
removal of calcium → Ca ATPase pump on SR AND plasma membrane Na/Ca exchanger
Blood Flow through the Heart
Path of Blood Flow (chamber/destination, valve)
vena cava → right atrium → tricuspid valve (atrioventricular) → right ventricle → pulmonary valve (semilunar) → pulmonary trunk → left and right pulmonary arteries → lungs (oxygenate blood) → pulmonary veins → left atrium → mitral valve/bicuspid valve (atrioventricular) → left ventricle → aortic valve → aorta → body
4 chambers
Left/right atria (superior)
Left/right ventricles (inferior)
4 Heart Valves
goal → promote unidirectional blood flow (only lets pass one way)
Atrioventricular valves → tricuspid, bicuspid/mitral
atria → ventricles
papillary muscles, chordae tendineae
Semilunar valves→ aortic, pulmonary
between ventricles and arteries
Vessels
Vena Cava
superior vena cava → drain deoxygenated blood from head and neck to heart
inferior vena cava → drain deoxygenated from lower body to heart
pulmonary artery → deoxygenated blood from right ventricle to lungs
pulmonary vein → oxygenated blood from lungs to left atria
Arteries → carry blood AWAY from heart
Veins → carry blood TOWARDS heart
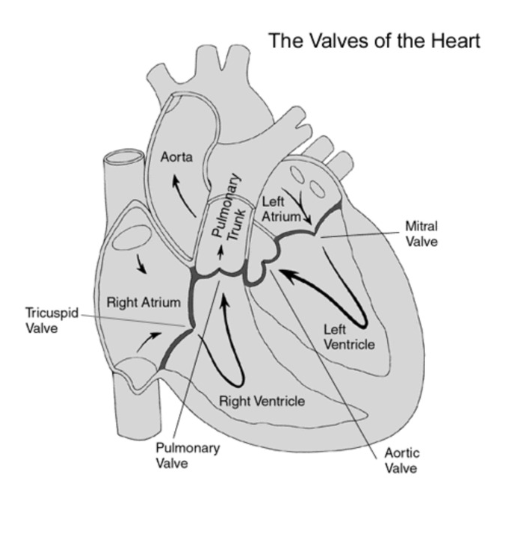
Order of valves mnemonic
Try Pulling My Aorta
Try → tricuspid valve
Pulling → pulmonary valve
My → mitral valve
Aorta → aortic valve
Pattern of Cardiac Muscle Contraction
contraction myocardium → sequence for efficient blood ejection
electrical signals trigger myocyte contraction
Conduction System → spread electrical signals in highly organized pattern
General events of cardiac contractions (Heartbeat)
deoxygenated blood returning to heart → AV valves (tricuspid and mitral) open, semilunar valves (pulmonary and aortic) closed
atria contract first so ventricles can “top off” fill with blood
ventricles contract (AV valves close to prevent backflow)
pressure builds in ventricles
semilunar valves open
blood ejected
Conduction System
Propagation of Electrical Signals
pathway of wave of electrical excitation
SA node (normal pacemaker)
atrial activation begin
→ Atrial Internodal fibers (atrial contraction)
spread signal across atrial conductile cells to AV node
spread to atrial contractile cells → atria contracts
→ AV node (slowed transmission of impulse→ allow ventricles to fill)
→ AV bundle/ Bundle of His (only electrical link between atria and ventricles)
signal to interventricular septum
→ Right and Left Bundle Branches
→ Purkinje Fibers (rapid propagation→ contract ventricles)
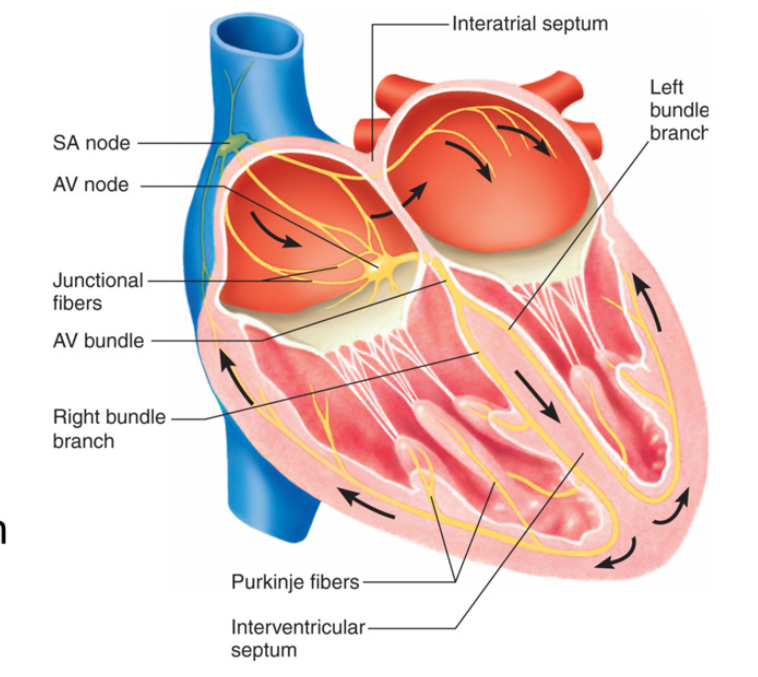
Conduction System
Sinoatrial Node and Atrioventricular Node
Normal Pacemaker of heart → SA Node (generates initial electrical signal)
Automaticity = ability to generate signals and contract on its own
spontaneous firing = 100/min (fastest rate of conduction)
overdrive suppression = the fast rate of firing of SA node suppresses other cells from acting as pace makers
prevent ventricles from contracting at the same time as atria
all conductile cells can generate signal → SA node has fastest rate → overrides automaticity of other conductile pathway → overdrive suppression
Atrial Internodal Pathway→ connects SA Node to AV Node
Specialized conducting cells
~50 msec to travel pathway
stimulus passed to contractile cells → spread across both atria → atria contract
stops at atria → myocardium of atria and ventricles are not connected
AV Node
specialized smaller conductile cells → slows electrical signal
100 msec to move through AV node
slow signals from atria to allow ventricles to fill with blood before contraction
normal firing rate 40/min
AV bundle/ Bundle of His enters interventricular septum
spread electrical signal from atria → ventricles
only electrical connection between atria and ventricles
Left and Right Bundle Branches
electrical signal travel towards apex along ventricles
left much larger → need to activate thicker muscle tissue in left ventricle → pumps blood to the whole body
Purkinji Fibers
larger cells → speed up electrical signal rate
fight conduction system
signal move up from apex → base
signal to contractile cells → contract ventricles → push blood upwards
normal firing frequency 15-20/min
if SA node not functioning → ectopic pacemaker → other pacemaker
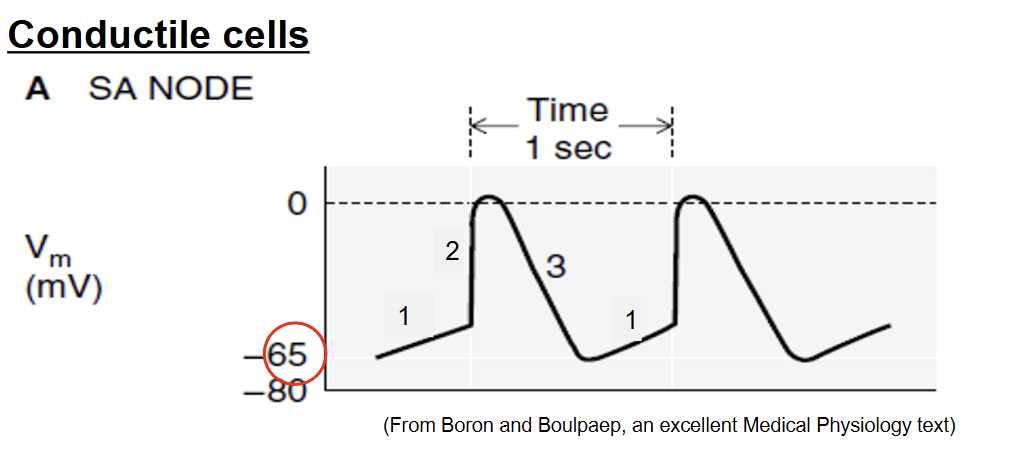
Action Potential: conductile cells (SA Node and AV node)
Phase 1: Pacemaker potential (no resting membrane pot)
Na+ leak channels are always open (rather than chemically gated channels → continuous increase of membrane potential)
Voltage gated channels closed
upward drift of membrane potential
no resting membrane potential → always gradually increasing → -65mV minimum
Phase 2: depolarization
threshold = -40mV
voltage gated Ca2+ channels open → large influx of Ca2+ → membrane potential spikes
Ca2+ plays role in contraction too → bind to troponin to move tropomyosin
Phase 3 - repolarization
voltage gated Ca2+ channels close → stop Ca2+ influx
Voltage Gated K+ channels open → K+ leaves cell
membrane potential decreases until -65 mV → start to gradually increase again
Ca2+ diffuse out gap junction→ neighboring atrial internodal fibers and contractile cells
Action Potentials: Contractile Cells
Phase 1: Rapid Depolarization
resting membrane potential (stable) = -90mV
Ca2+ from neighboring cells enter → increase membrane potential Threshold = -75 mV
quick opening of voltage gated Na+ channels → rapid Na+ influx
Membrane potential increases rapidly
Phase 2: Plateau
Early repolarization
voltage gated Na+ channels close
Plateau
Voltage gated Ca2+ channels open (long/L type CA2+ channels → open for long time)
→ slow calcium influx throughout entire period
slow K+ efflux + slow calcium influx → plateau
Ca2+ channels close staggered → no clear repolarization point
Ca2+ also binding to troponin to move tropomyosin
Phase 3: Repolarization
Voltage gated Ca2+ close
Voltage gated K+ channels open → K+ leaves cell
membrane potential decreases
Ca has 2 functions → aid in contraction (bind to troponin) and bind to ryamodine receptors to release more Ca
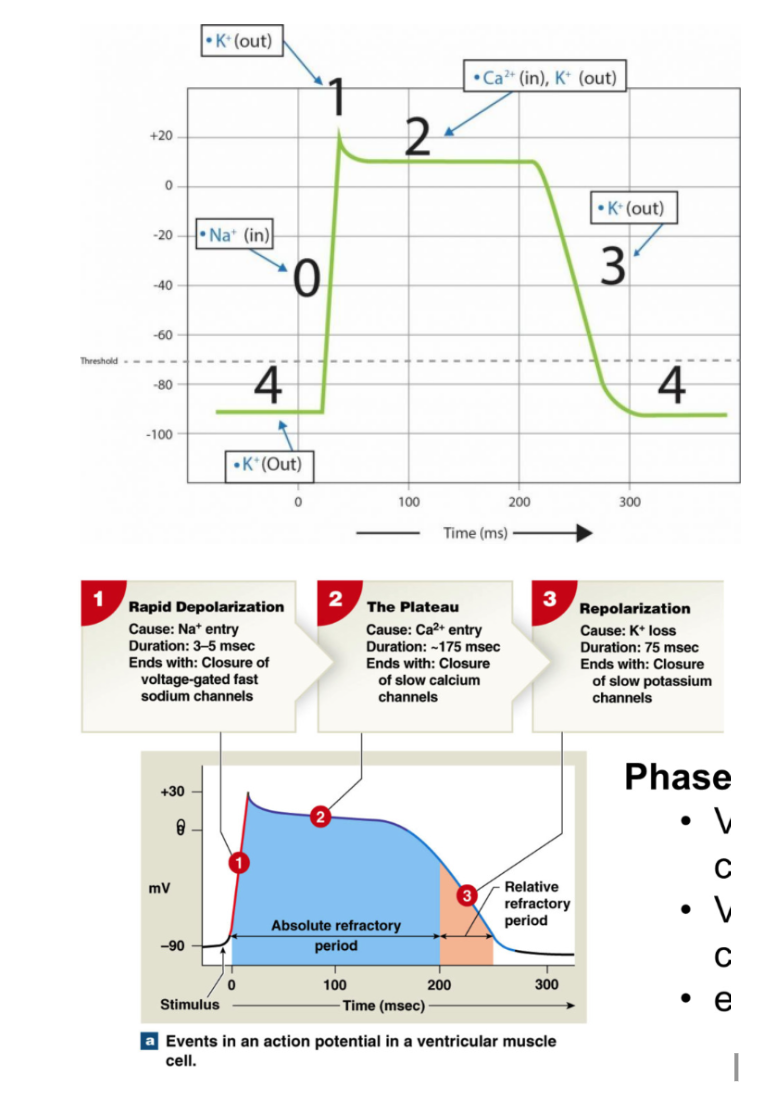
Refractory Periods: Contractile cells
Absolute refractory period: LONG compared to skeletal muscle (200 msec)
no additional action potential at all
includes: depolarization, plateau, and initial period of rapid repolarization
because Na+ channels are open during depolarization and Ca2+ open during plateau → all channels doing all they can already
Relative refractory period
difficult to initiate action potential → require more signal
includes: remaining repolarization
Together → limit frequency of action potentials
prevent tetanic contractions (early contraction) → don’t want to mess up timing
prevent ectopic pacemaker from stimulating contraction (any pacemaker other than SA node)
→ allows time for ventricles to fill
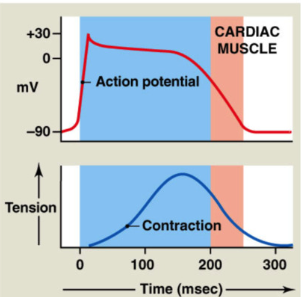
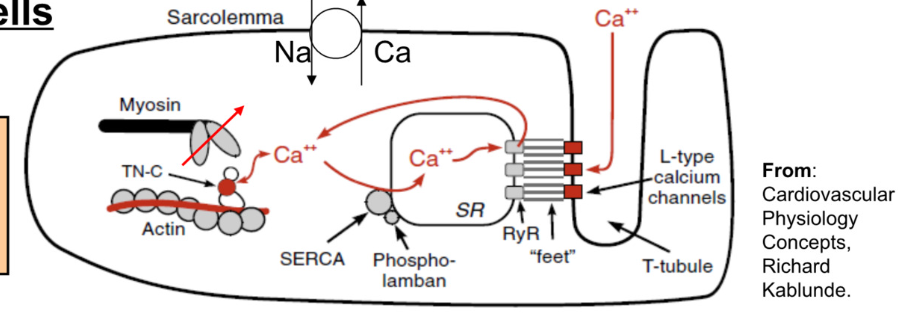
Excitation-Contraction Coupling: contractile cells
Contractile cells
Calcium required for contraction:
80% from SR (sarcoplasmic reticulum)
20% from ECF
Increase in Cytosolic Calcium
Calcium enters myocyte via L-Type calcium channels
this calcium binds to ryanodine receptors on SR → stimulates release of calcium from SR
Calcium Induced Calcium Release → Ca2+ signals SR to release more Ca2+
Removal of Cytosolic Calcium (during relaxation)
calcium pumped back into SR (SERCA pump → Sarcoplasmic Endoplasmic Reticulum Calcium Pump)
calcium moved to ECF via Na/Ca exchanger
Ca has 2 functions → aid in contraction (bind to troponin) and bind to ryanodine receptors on SR to release more Ca
Autonomic Regulation of SA node (conductile): increasing heart rate
SA node signal 100/min normally
sympathetic nerves → synapse on SA node → influence activity
heartrate increased by sympathetic nervous system via norepinephrine binding to Beta-1 receptors
Sympathetic regulation: norepinephrine → Beta-1 Receptors on SA node → increase heartrate
increased opening of Na+ and Ca 2+ ion channels → more influx of Na+ and Ca 2+
reduced repolarization → builds up more positive charge → steepens pacemaker potential (less charge difference from pacemaker potential → threshold)
effect: shorter time for SA node to reach threshold → increase heart rate
POSITIVE CHRONOTROPIC EFFECT: INCREASES HEART RATE
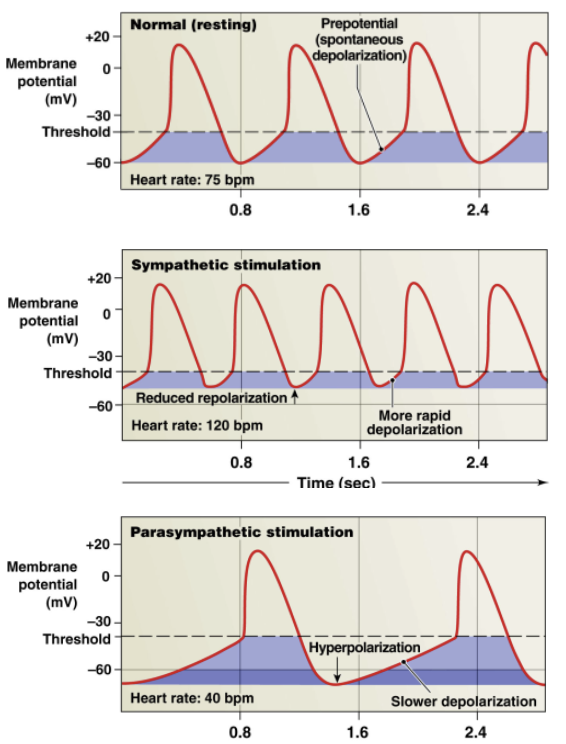
Autonomic Regulation of SA node: decreasing heart rate
Parasympathetic Regulation: ACh → Muscarinic Receptors on SA node
increased opening of K+ channels
efflux of K+ → lose more positive charge (larger charge difference from pacemaker pot → threshold)
hyperpolarization → decreases steepness of pacemaker potential
effect: longer time for SA node to reach threshold → decrease heartrate
NEGATIVE CHRONOTROPIC EFFECT: DECREASES HEART RATE
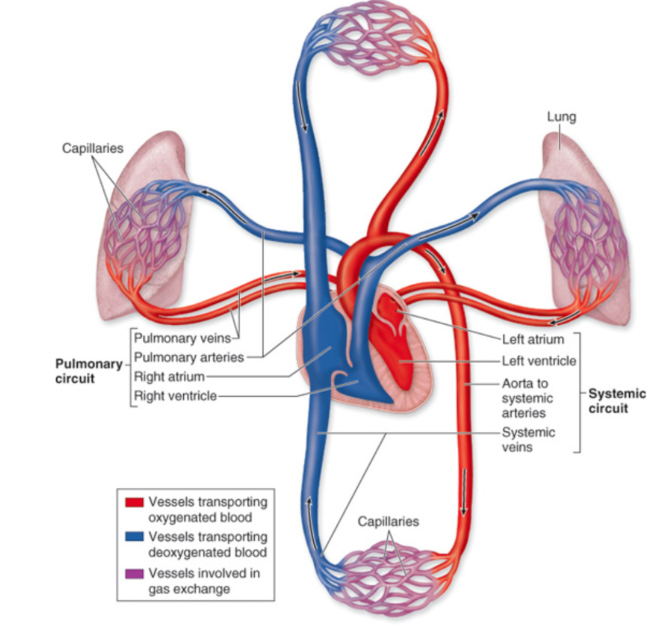
Blood Flow
right side of heart → pump deoxygenated blood to lungs
left side of heart → pump oxygenated blood to body
Pulmonary circulation
vessels carrying blood to and from lungs
Systemic circulation
vessels (arteries and veins carrying blood to and from body
Oxygenated Blood
pump to body
Deoxygenated blood
pump to lungs
Cardiac Cycle
electrical and mechanical events that repeat with every heart beat
duration (s/beat) = (60 sec/min)/ Heart Rate (beats/min)
Systole: contraction
Diastole: Relaxation
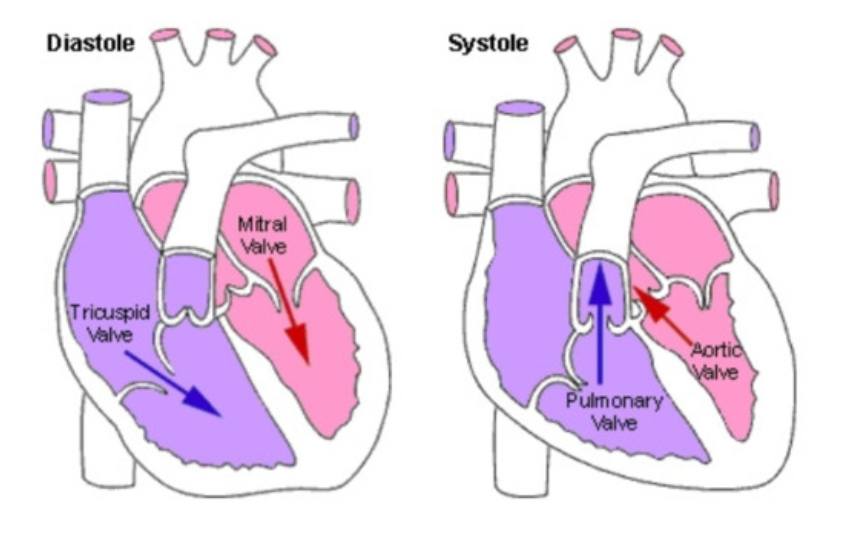
Cardiac Cycle
22% atria in systole (contract)
88% atria in diastole (relax)
1/3 ventricle in systole
2/3 ventricle in diastole
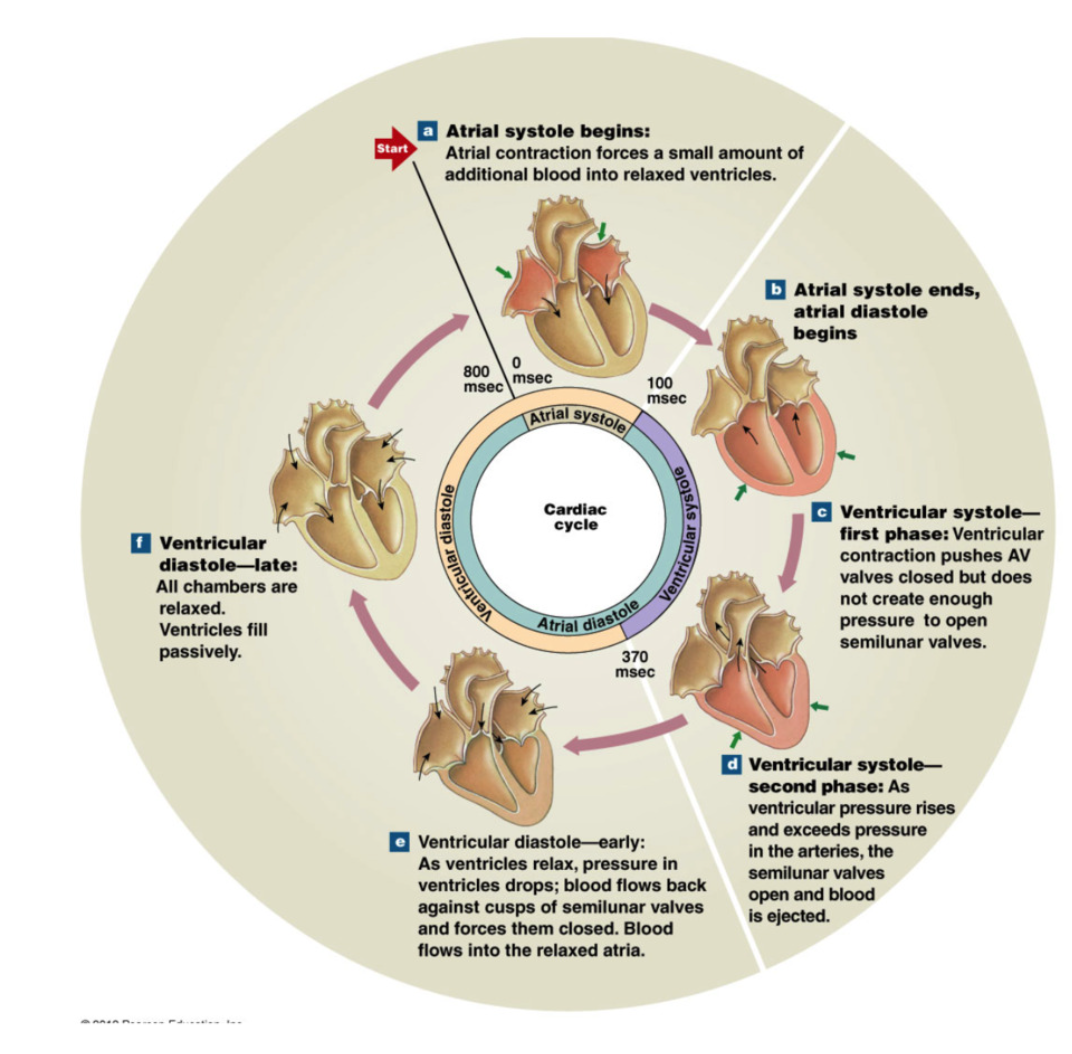
Ventricular Diastole and Atrial Systole
relaxation and filling of ventricles with blood
atrial contraction
SIMILAR EVENTS OCCUR ON RIGHT AND LEFT SIDE
BLOOD FLOWS FROM HIGHER TO LOWER PRESSURE
ventricles just ejected blood to arteries → empty ventricles
Early Diastole → ventricles relax
pressure drops
isovolumetric relaxation: all heart valves closed (no blood volume movement) → pressure less than arteries, but more than atria
Artery (aorta and pulmonary trunk) pressure > Ventricular pressure (because ventricles just pumped out blood) → semilunar valves (pulmonary + aortic) closed
Ventricular pressure > Atrial pressure → AV valves (tricuspid + mitral) close
Late Diastole → continued ventricular relaxation
ventricular pressure continues to drop
Ventricular pressure < Atrial pressure → AV valves open
blood flow from atria → ventricles
Rapid Ventricular Filling: “Passive filling” → 70% of blood pours into ventricles via pressure difference (blood travels from high → low pressure) and gravity
Atrial Systole
ventricles “topped off” → push remaining 30% of blood into ventricles via atrial contraction
End Diastolic Volume → approx 150mL blood per ventricle
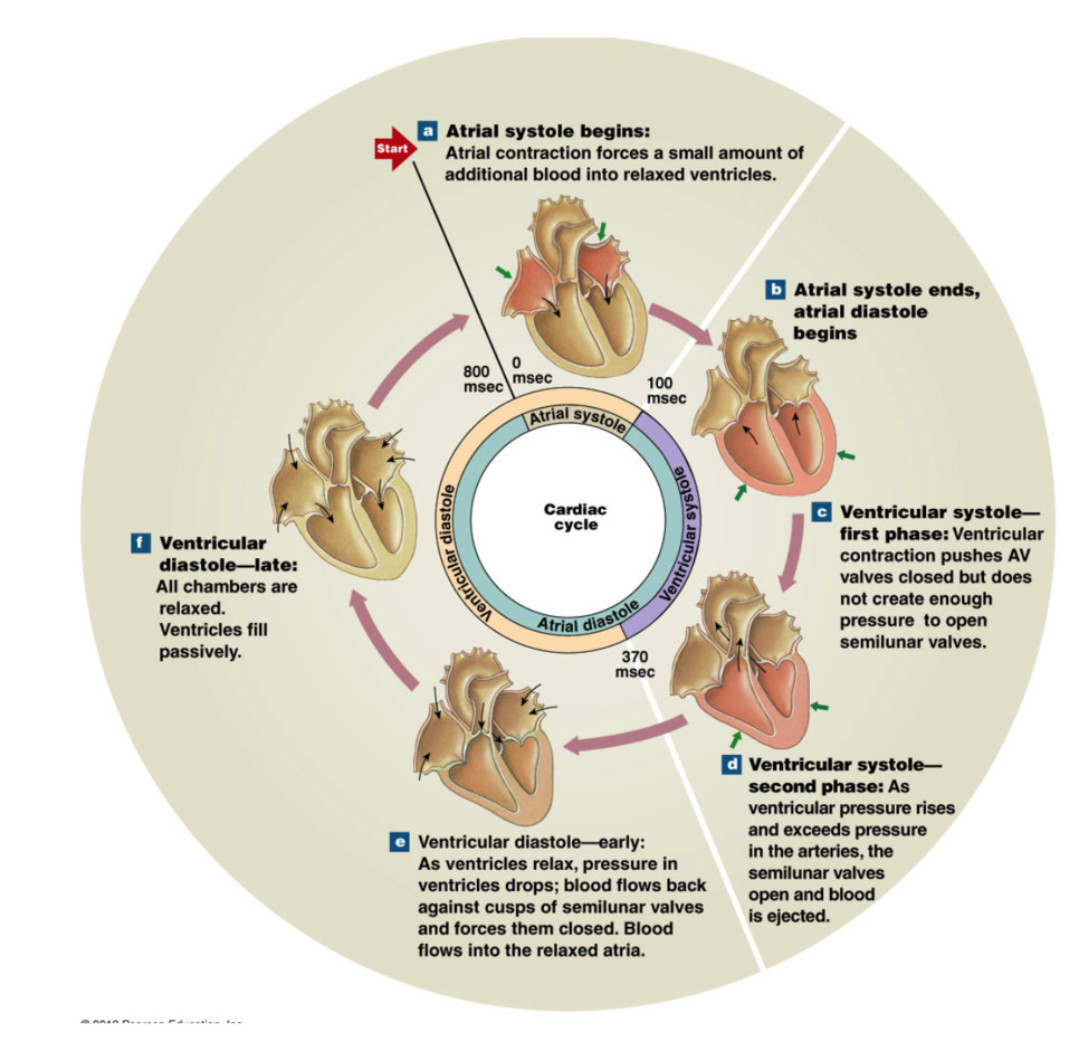
Ventricular Systole
contraction of ventricles
electrical signal by conductile cell purkinje fibers (apex up to→base) → contraction of ventricles up
BLOOD FLOWS FROM HIGH TO LOW PRESSURE
ventricles are full of blood now
1st Phase
begin as atrial systole ends
ventricles begin to contract → increase ventricular pressure
Ventricular pressure > atrial pressure → AV valves close (1way valve)
artery pressures > ventricular pressure → SL valves closed
Isovolumetric contraction: ventricle pressure increase → all heart valves closed
pressure rises steeply until → ventricular pressure > aortic pressure (artery) → SL valves open
2nd Phase
Semilunar valves open → blood ejected from ventricles to arteries
Rapid ejection: ventricular pressure continues with big decrease in ventricular volume (big squeeze → blood ejected)
Reduced ejection: less rapid ejection in ventricular volume (less pressure) → ventricular and aortic pressure begin to fall
Stroke Volume → approx 70mL blood ejected per ventricle
End-Systolic-Volume → approx 80mL remaining in ventricle
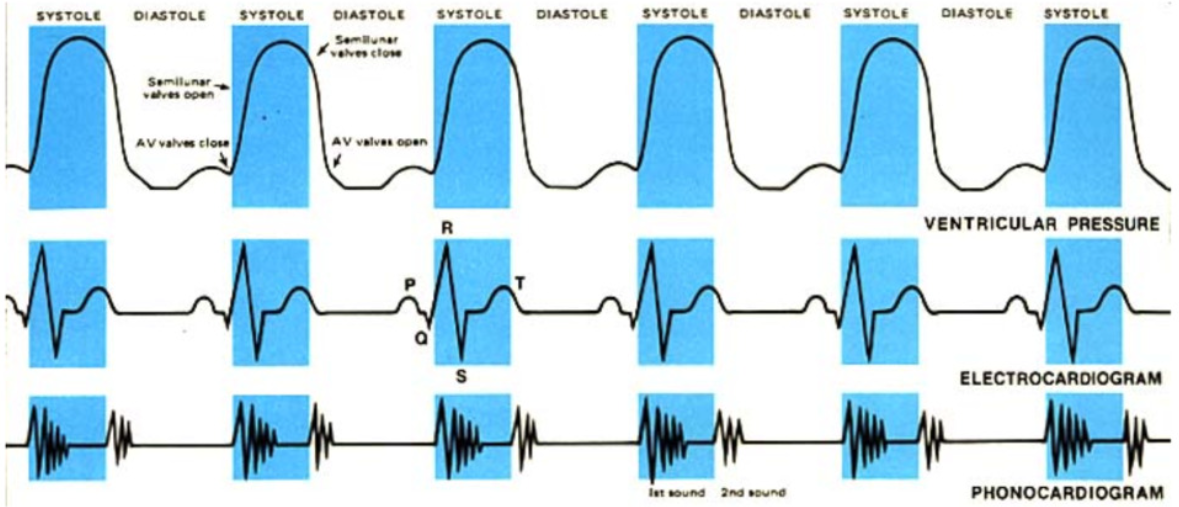
Heart sounds
auscultation: listening to internal body sounds
S1 “lubb” → closure of AV valves
sound from blood hitting closed valve
S2 “dubb” → closure of semilunar valves
early ventricular diastole
murmur → regurgitation of ventricular blood back into atria
slight backflow→ malformed AV valve
Bruit → abnormal sound as blood runs past obstruction through arteries
ECG →
P wave
atrial depolarization → atrial systole
at end of ventricular diastole
QRS complex
ventricular depolarization → ventricular systole
T wave
ventricular repolarization → end of systole →beginning of ventricular diastole
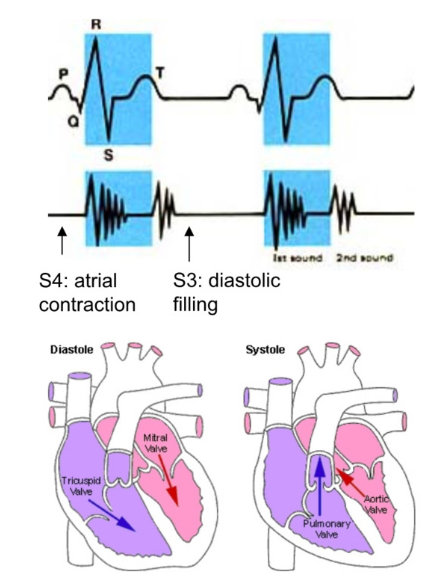
Overlapping Electrical and Mechanical Activity e
Ventricular Systole
QRS wave → ventricular depolarization → contraction
AV valve close when ventricular pressure > atrial pressure (1st heart sound) → both valves closed (artery pressure > ventricular pressure → SL valves also closed)
isovolumetric contraction → increase in ventricular pressure > artery pressure → SL valves open
ventricular ejection
Ventricular diastole
T wave → ventricular repolarization → end of systole → relaxation
diastole when t wave is complete
drop in ventricular pressure < aortic pressure → aortic valve close (second heart sound
fall of ventricular pressure < arial pressure → AV valve opens
P wave → atrial depolarization → atrial contraction → small rise in ventricular pressure
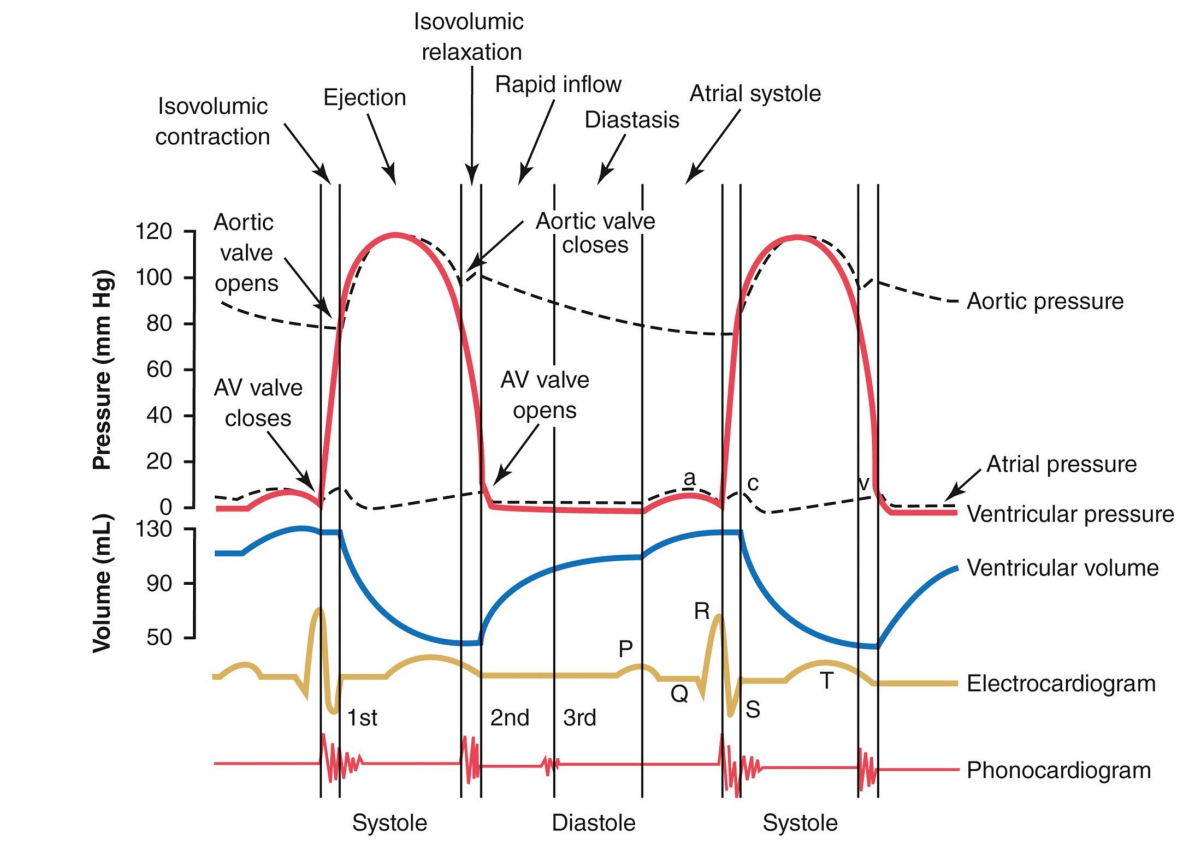
Wigger’s Diagram (for left ventricle)
Ventricular Systole: QRS → ventricular contraction
immediately after atrial contraction→ topping off → immediate rise of ventricular volume → increase in ventricular pressure > atrial pressure → AV mitral valve closes → 1st heart sound
isovolumetric contraction w/ 2 closed valves (ventricular pressure < aortic pressure→ aortic valve still closed) → sharp increase in pressure, no volume change
rapid ejection: ventricular pressure > aortic pressure → aortic valve opens → rapid decrease in ventricular volume and pressure
reduced ejection: T wave → repolarization of ventricles → lower rate of decrease in volume and pressure
Ventricular Diastole (completion of repolarization): end of T wave → diastole start
isovolumetric relaxation: decrease in ventricular pressure < aortic pressure → aortic valve close → 2nd heart sound
both valves closed (ventricular pressure > atrial pressure → AV valve closed) → rapid decrease in pressure, no volume change
Rapid refilling: drop in ventricular pressure < atrial pressure → AV mitral valve opens → 70% of blood passively pours into ventricle → large increase in volume
Atrial Systole (during ventricular diastole): P wave → atrial contraction
slight increase in atrial/ventricular pressure (AV valves are open)
atrium contract → slight bump in ventricular volume → last 30% topping off ventricle
Cardiac Output
Cardiac output: amount of blood pumped by each ventricle in one minute
Cardiac output = (heart rate) x (stroke volume) = HR x (EDV - ESV)
stoke volume = volume of blood ejected every contraction
affected by preload, contractility, afterload
EDV = end diastolic volume
max blood volume filling ventricle during diastole
ESV = end systolic volume
remaining blood remaining in ventricle after contraction
Stroke volume = EDV-ESV
volume of blood ejected from ventricles
contractility
force of heart muscle contraction
more force → more blood ejected
preload
degree of stretch of cardiomyocytes at the end of ventricular filling/diastole → use EDV to measure
afterload
resistance ventricles must overcome to eject blood
ventricular pressure > SL pressure
Altering Heart Rate
autonomic nervous system: cardiac centers of medulla oblongata
Sympathetic innervation
positive chronotropic effect → increase HR → increase cardiac output
norepinephrine → Beta-1 receptors → opening of Na+ and Ca2+ channels
Parasympathetic innervation
negative chronotropic effect → decrease HR → decrease cardiac output
ACh→ muscarinic receptors → openning K+ channels
venous return
volume of blood veins return to heart
direct effect on SA node
larger volume of blood → stretch atria → stretch SA node fiber → increase rate of depolarization → increase HR
Altering Stroke Volume LEARN BETTER
dependent on EDV (end diastolic volume) and ESV (end systolic volume
increase stroke volume → increase cardiac output
increase heartrate → increase cardiac output
Factors influencing EDV → preload
preload = heart muscle stretch when ventricles are full → EDV
filling time → dependent on HR
longer fill time → EDV larger
increase in HR → shorten filling time → increase CO
body position and activity level
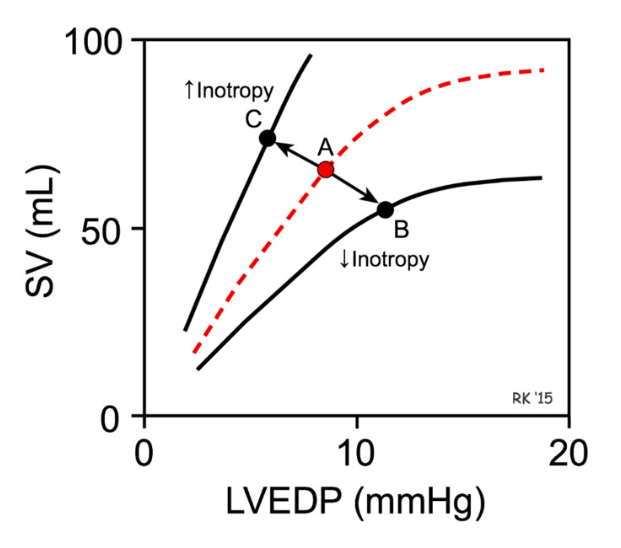
Starling’s Principle of the Heart: higher preload → higher stroke volume
greater preload (stretch of cardiomyocytes during end of ventricular diastole) = higher EDV (more ventricular filling) → greater the contraction
related to tension levels produced in muscle
more blood in heart → potential to eject more blood
Factors influencing ESV (end systolic volume):
afterload: ventricular pressure required to open semilunar valves (ventricular pressure > SL pressure)
proportional to amount of pressure present in aorta at time of ventricular contraction
amount of blood pressure present in aorta at time of contraction (HR)
if aorta is full, more pressure is required to open SL valves → decreased stroke volume and vice versa
vasodilation → vessels widen→ less pressure in aorta → lower afterload → easier to pump blood
vasoconstriction → vessels narrow → more pressure in aorta → higher afterload → more difficult to pump blood
contractility: amount of force produced during contraction
dependent on inotropes: factors that influence contractility (dependent on levels of cytosolic calcium 80% from SR, 20% from ECF)
Starling’s Principle of the Heart
strength of ventricular contraction increases with an increase in preload
increased preload (more ventricular stretch) → higher EDV (ventricles filled with more blood) → more contractile force (like a filled balloon)
linear relationship between EDV and Stroke volume
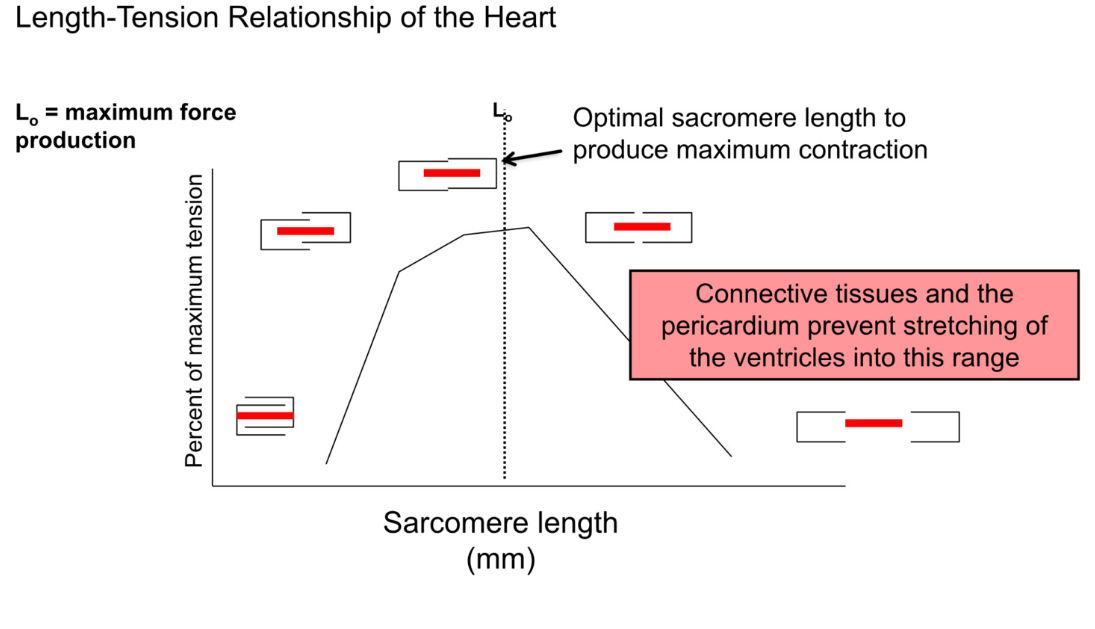
Length-Tension relationship of heart
contraction is dependent on overlap between actin and myosin
optimal overlap → maximum contraction → best stroke volume
when EDV is too low (empty volume) → cells not stretched → too much overlap of actin and myosin → bad contraction
as ventricles fill → better actin myosin overlap → better stroke volume
connective tissues and pericardium prevent overstretching
Contractility
increased strength of contraction of ventricle due to increases in cytosolic calcium
positive inotropic effect → increase Ca2+ levels → increase contractility
negative inotropic effect → decrease Ca2+ levels → decreased contractility
because more Ca2+ → more troponin binding → more free tropomyosin → more contraction
Factors affecting cytosolic calcium
heart rate
during action potential of contractile cell → Ca2+ into cell
more time between act pot → time to clear Ca2+
increase heart rate → more Ca2+ leftover
Size of inward Ca2+ current during plateau of contractile myocyte action potential
larger concentration difference between ECF and cytoplasm → higher rate of movement into cell
SNS phosphorylates L-type Ca2+ channels → more efficient at moving Ca2+ into cell
Amount of Ca2+ stored in SR
thyroid hormone → acts as transcription factor → increase transcription of SERCA (sarcoplasmic endoplasmic reticulum Calcium ATPase) → more Ca2+ into SR→ more Ca2+ out of SR (calcium induced calcium release)
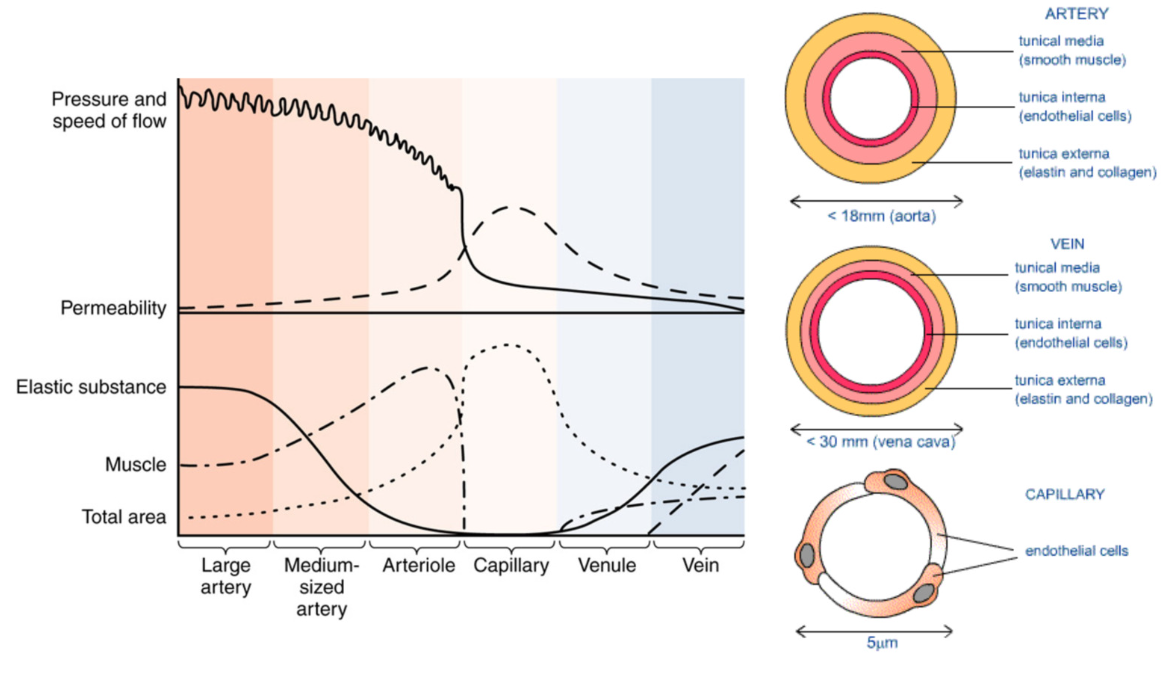
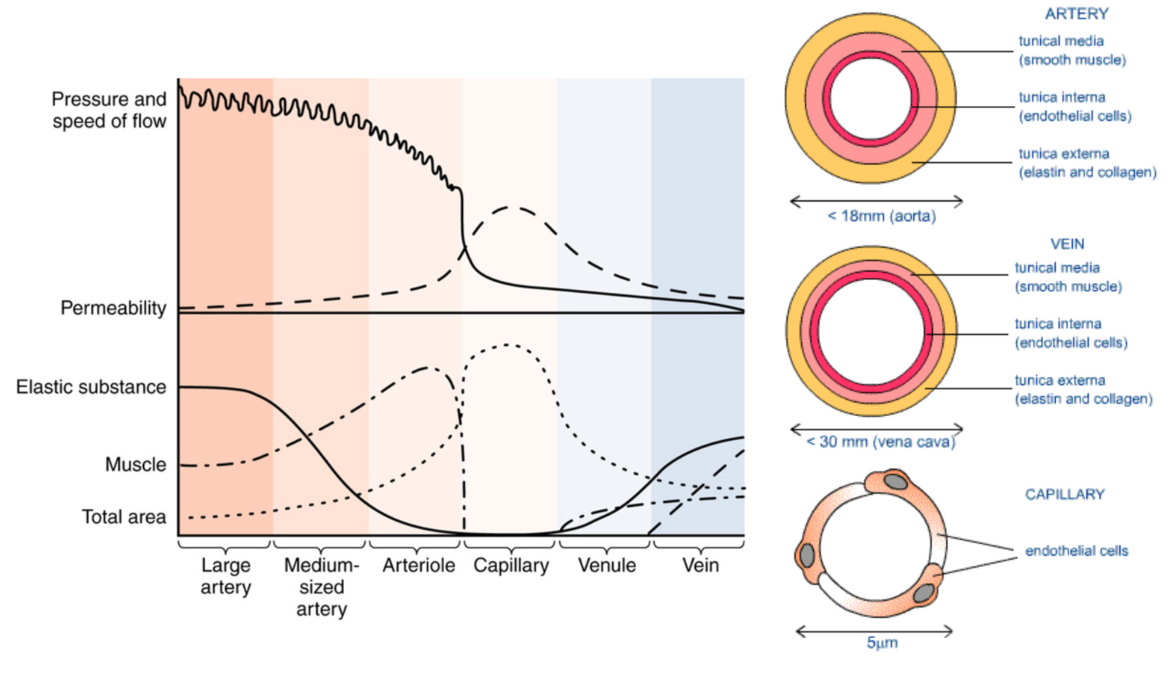
Vascular Wall (blood vessel)
lumen → space
Tunica intima (closest to lumen)
endothelium
epithelial cells → basement membrane separates intima from media
Tunica Media
smooth muscle cells → contract and determine diameter of vessel
elastic fibers → flexibility
Tunica Adventitia
connective tissue
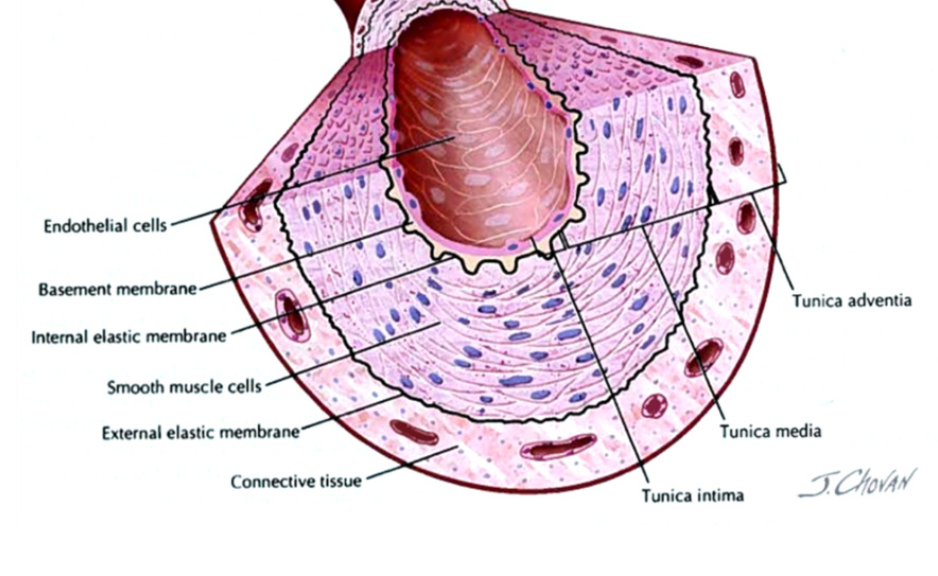
Tunica Intima: Endothelium
specialized epithelium (simple squamous)
function: barrier and filtration → control what moves between blood and interstitial fluid
plasticity:
help new vessel growth (angiogenesis) in response to injury and ischemia (lack of blood flow)
secretory: regulate neighboring smooth muscle
vasodilators: nitric oxide (NO) and prostacyclin
vasoconstrictors: endothelin
anti-aggregatory for platelets
Tunica Media → Smooth Muscle
contractile cells → contraction and relaxation
Vascular tone
baseline level of contraction
Vasomotion
change in caliber (diameter) of blood vessel
contraction influenced by signals by endothelium/ nervous system
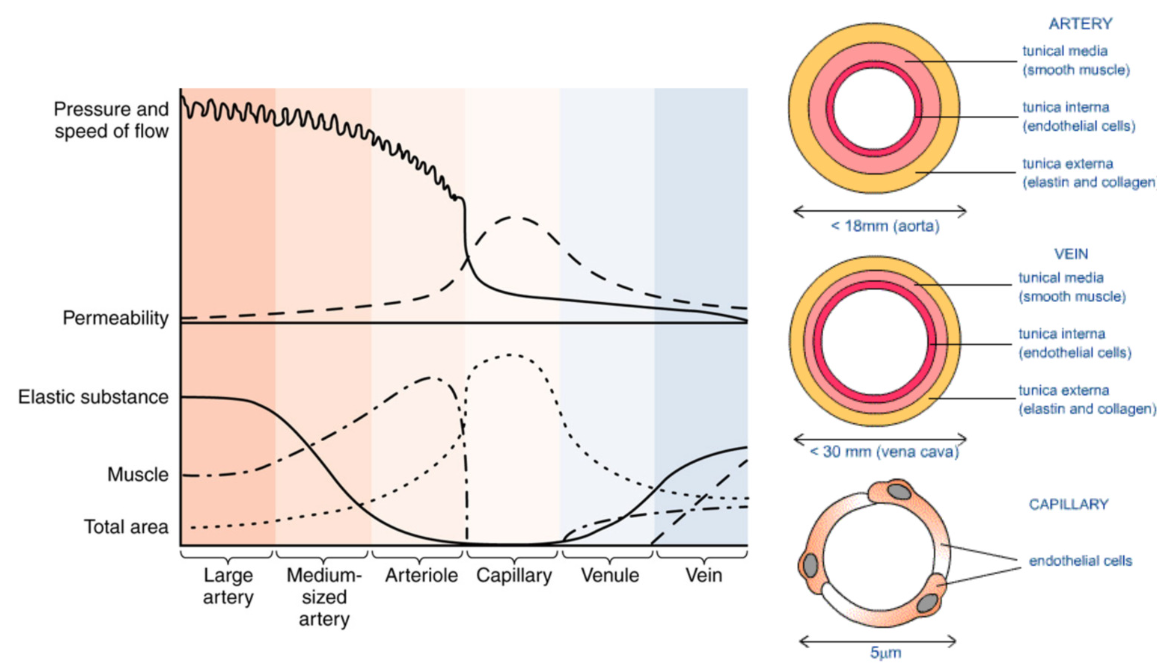
Heterogeneity of Vessels: different types
Artery
carry blood away from heart
thick walls
most elastic tissue
most smooth muscle
highest pressure
large arteries branch into → small arteries (arterioles) → capillaries → capillary beds
lowest permeability between blood and ISF
most smooth muscle in arterioles
blood flow via smooth muscle contraction
Structure: round, relatively thick wall
Tunica intima→ endothelium (rippled due to
vessel constriction), internal elastic
membrane (present)
Tunica media→ thick, smooth muscle cells,
and elastic fibers, external elastic membrane
(present)
Tunica externa→ collagen and elastic fibers
Function: Arteries move blood AWAY from
the heart
NO VALVES!
HIGHEST PRESSURE, MOST ELASTIC SUBSTANCE
LEAST PERMEABLE, LOWEST TOTAL SURFACE AREA)
Vein
carry blood towards heart
small veins (venules) → larger veins
less smooth muscle
less elastic tissue
lumen of vein larger > artery
blood flow via surrounding skeletal muscle contraction
Structure: flattened or collapsed, relatively
thin wall
Tunica intima→ endothelium (smooth)
Tunica media→ thin, lots of smooth muscle
cells and collagen fibers
Tunica externa→ collagen and elastic fibers,
smooth muscle cells
NO INTERNAL OR EXTERNAL ELASTIC
MEMBRANES
Function: Veins move blood TOWARDS the
heart
HAVE VALVES!!
Capillary
site of product exchange
allow for nutrient/oxygen and waste exchange between the blood and surrounding cells
only endothelium without tunica media or tunica adventitia
narrow lumen
low pressure
connect to arteries and veins
highest permeability
GREATEST TOTAL SURFACE AREA, MOST PERMEABLE
(Additionally, LEAST MUSCLE + ELASTIC TISSUE… bc only consist of tunica intima)
Veins and Arteries connect at Capillary beds
Arteries
smaller lumen (hole)
thick tunica media → thick smooth muscle walls → control over diameter
abundant elastic fibers
high pressure
low permeability
blood flow via smooth muscle contraction in tunica media
no valves
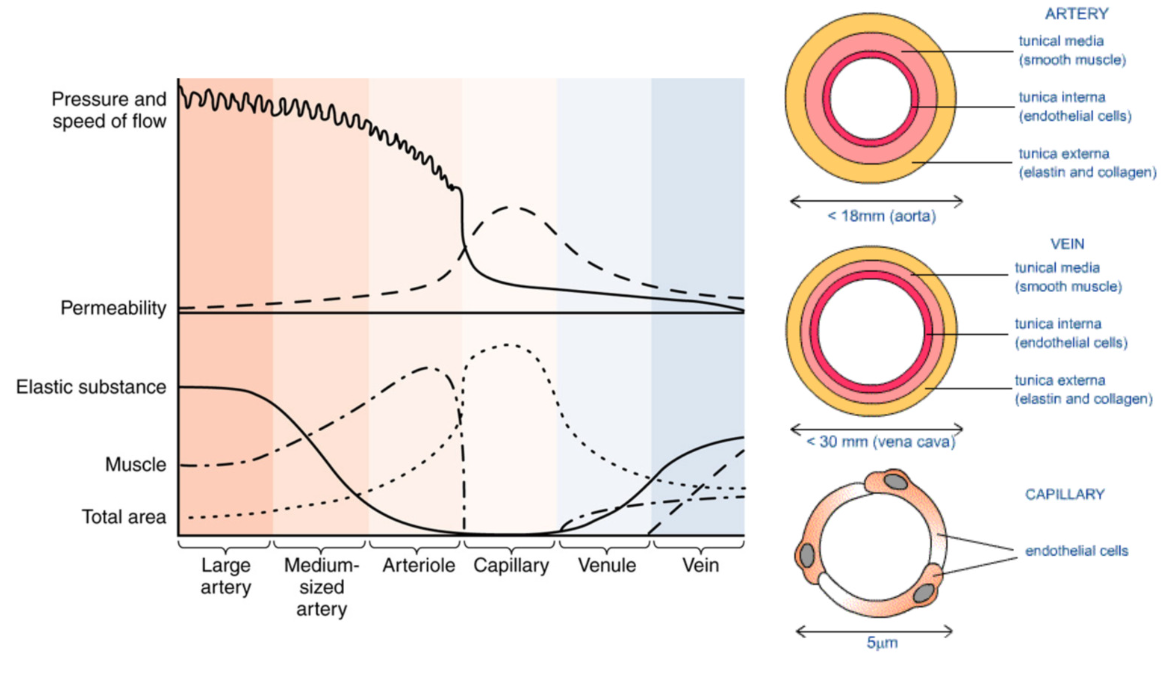
Veins
less tunica media → less muscle contraction
less elastic fibers than arteries
more permeability than arteries, far less than capillaries
blood flow via surrounding skeletal muscle contraction
has one way valve system
pressure higher on one side → valve open → pressure higher on other side → valve closed
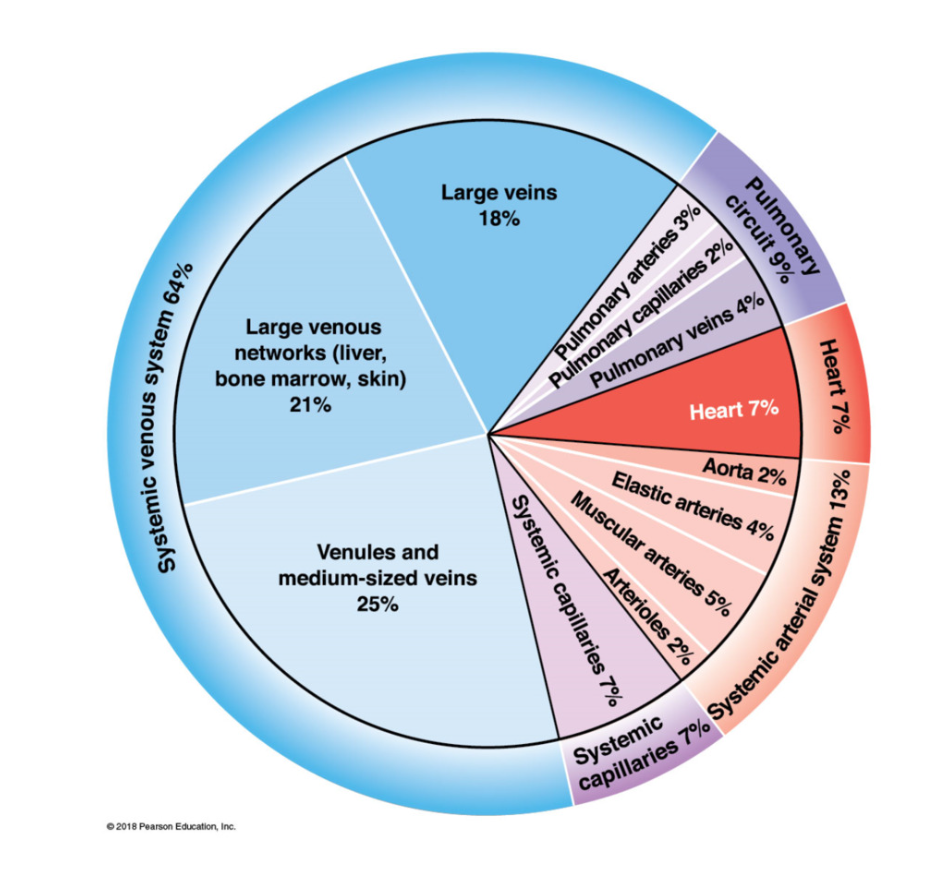
Blood distribution
70% blood in venous system
7% blood in heart
7% in capillaries
13% in arteries
Capillaries
capillary structure
endothelial tube inside thin basement membrane
no tunica media or adventitia
diameter ~ 1 RBC
higher permeability than veins and arteries
higher pressure than veins, far less than arteries
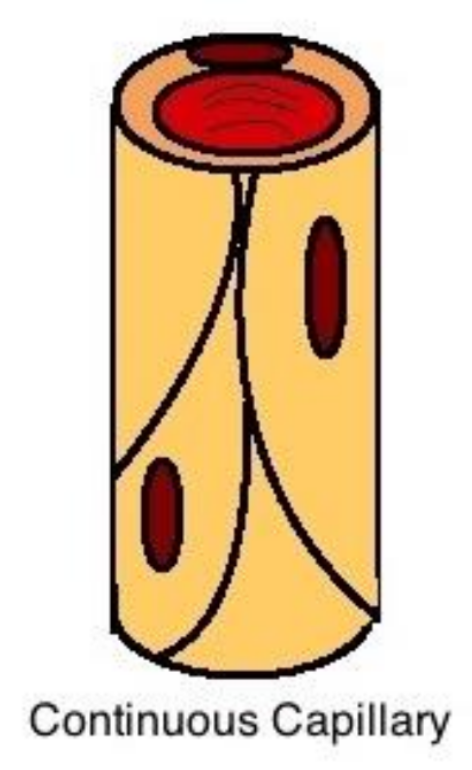
Continuous Capillaries
continuous capillaries:
little permeability
found in all tissue except epithelia and cartilage
complete endothelial lining → endothelial cells packed tightly
allow diffusion of:
water, small solutes, lipid soluble materials
prevent diffusion of:
blood cell and plasma protein
specialized tight continuous capillaries in CNS and thymus (BBB)
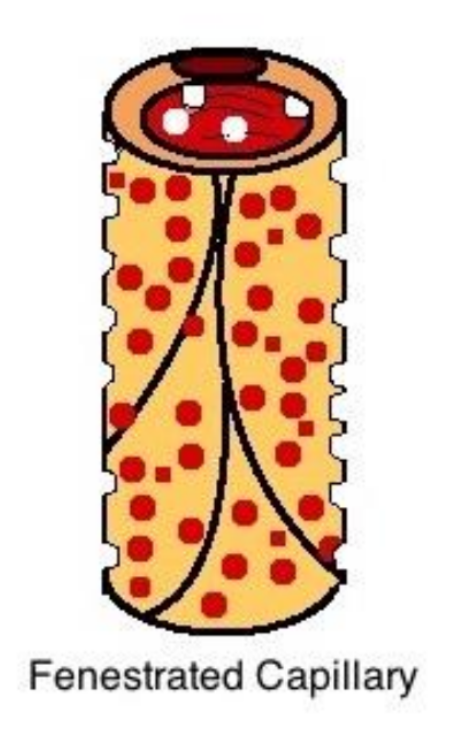
Fenestrated Capillaries
Fenestrated capillaries
greater permeability
pores in endothelial lining
allow rapid exchange of water and larger solutes
found in
choroid plexus → nutrients into CSF
endocrine organs → hormones into blood
kidneys → filter blood
intestinal tract → absorb nutrients
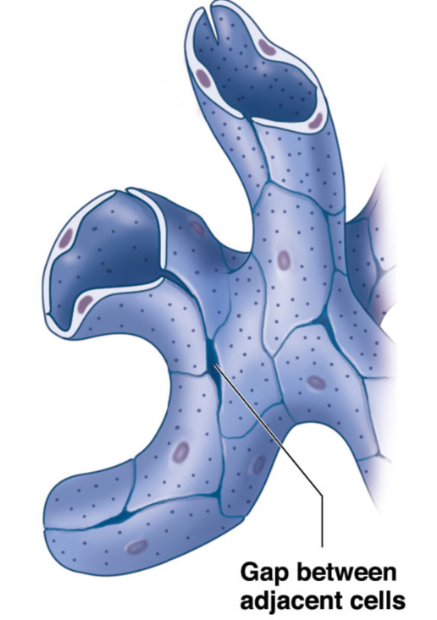
Sinusoidal capillaries
sinusoids
greatest permeability
gaps between adjacent endothelial cells
allow free exchange of water and large plasma proteins
found in
liver
spleen → passing of RBCs
bone marrow →blood cells into blood stream
phagocytic cells monitor blood at sinusoids
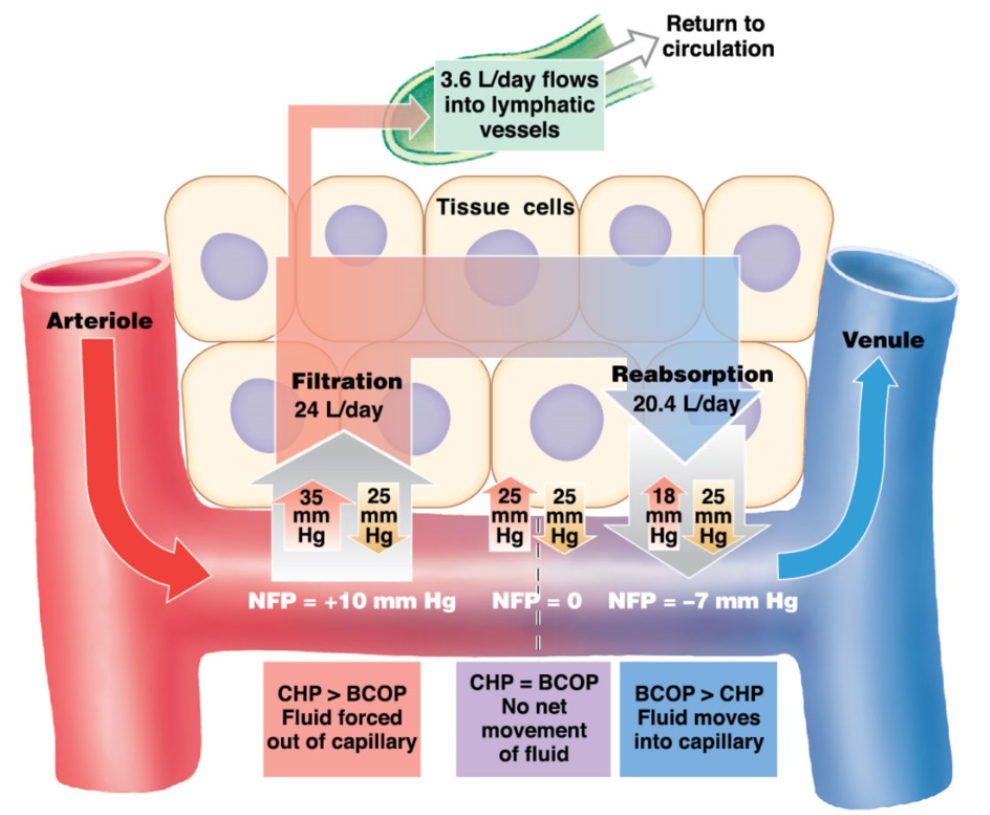
Starling Forces (for blood) RELEARN !!!!
movement of fluid in capillary beds
Blood colloid osmotic pressure (BCOP) → osmolarity of blood
solute move from high → low concentration
arteries and veins have similar blood osmolarity (similar amount of albumins) → because albumins are plasma proteins and can’t usually pass through capillaries
Capillary hydrostatic pressure (CHP) → volume of blood
water move from high → low concentration
arterial end: CHP>BOP
arteries have higher pressure → CHP pushing fluid out overcomes BCOP drawing fluid in
filtration → push fluid out of capillary
venule end: CHP < BOP
venules have low pressure → the BCOP drawing fluid in overcomes CHP
reabsorption → bring fluid into capillary
Microcirculation
Flow through a region is determined by pressure and resistance of microcirculation
Key terms Circulation
Blood Flow (Q)
volume of blood flowing through vessel per unit of time (eg capillary, organ, system)
Resistance (R)
force opposing flow
vascular resistance determined by diameter and length of vessel
Total peripheral resistance (TPR)
resistance of entire cardiovascular system
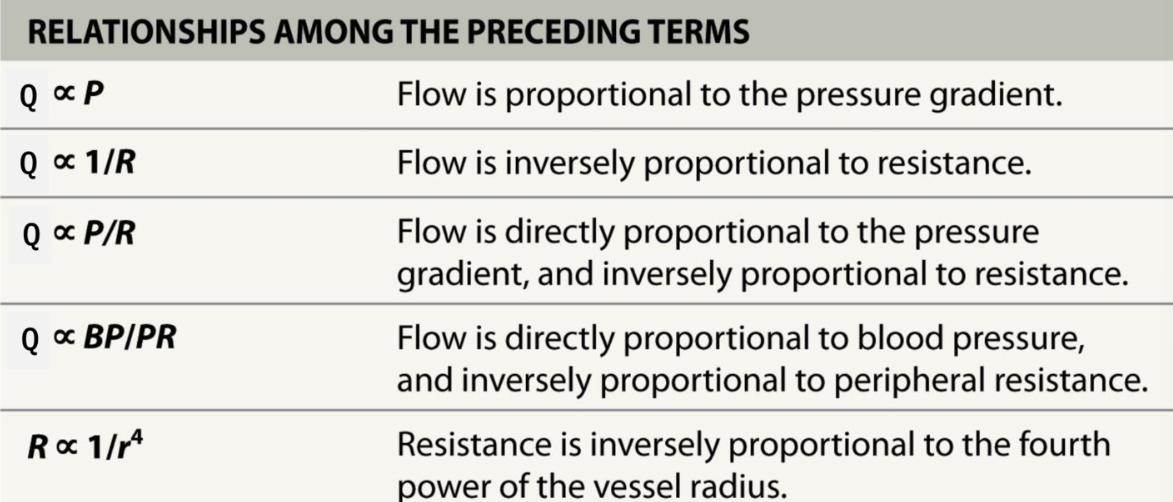
Blood flow Relationships
Q ∝ P
Q ∝ 1/R
Q ∝ P/R
Q ∝ BP/PR
R ∝ 1/ r4
Blood Flow
Q = ΔP/R
blood flow from high → low pressure
pressure difference high → blood flow high
pressure difference low → blood flow low
resistance prevents blood flow
resistance high → blood flow low
resistance low → blood flow high
Blood Pressure
Blood pressure:
measure of force of circulating blood exerted on arterial walls during systolic and diastolic heart pulses
arterial pressure highest and most dynamic
normal - 110mmHg/70mmHg (systole/diastole)
dependent on
cardiac output (volume of blood)
vasomotion (size of vessel)
vasodilation → BP decrease
vasoconstriction → BP increase
Blood pressure regulation systems overview
Autoregulation (local level) → 1st line of defense
vasodilators/vasoconstrictors
at tissue level
sphincter control in capillary beds
Neural Regulation → 2nd line of defense
Cardiovascular centers
vasoconstriction and vasodilation
baroreceptor reflex
chemoreceptor reflex
Hormonal Regulation → long term effects
Renin-Angiotensin II - Aldosterone System (RAAS)
immediate/long term effects
Atrial Natriuretic Peptide (ANP)
long term regulation of ECF volume
opposite effect of RAAS
Autoregulation: local blood flow
2 mechanisms regulating blood pressure locally
endothelium:
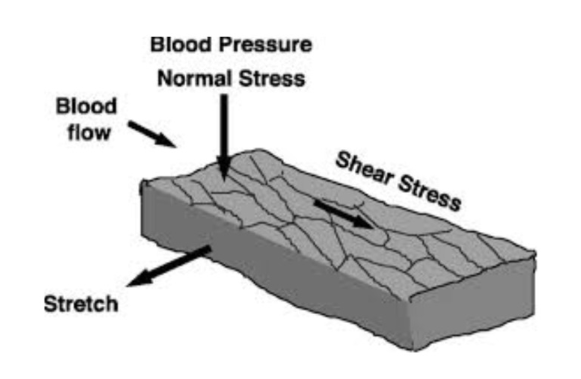
in response to friction (shear stress) → release vasodilators (NO and prostacyclin)
too much pressure → friction/sheer stress → release vasodilators to reduce pressure → sent to smooth muscle
smooth muscle:
in response to excessive stretch → smooth muscle constricts → myogenic regulation
important to maintain systemic flow in relation to gravity and body position
sudden change in pressure (eg stand up → blood rush down → arteries stretch → arteries constrict (push blood back up) → increase BP in upper body
vasomotion
at rest precapillary sphincters normally open/close
vasodilators → in response to abnormal tissue constriction → trigger vasodilation → higher rate of capillary closing
eg decreased O2, increased CO2
vasoconstrictors → thromboxane (reduce flow to damaged vessel), prostaglandins (pain), endothelin (released by endothelium
neural regulation
Cardiovascular control center (medulla oblongata)
vasomotor center:
directs vasomotor responses in blood vessels
cardiac centers:
cardioacceleratory center → increase cardiac output via SNS
cardioinhibitory center → decrease cardiac output via PNS
Supramedullary regulation
hypothalamus and cortex connect with cardiovascular control center to alter its activity → eg during exercise, emotional response, etc
Change in blood flow locally vs systemically
Q = P/R → increase pressure → increase resistance
Systemic blood flow (through whole system)
constrict artery → increase pressure → increase blood flow
pressure is more important systemically
Local blood flow
dilate vessels → decrease resistance → more blood flow
resistance more important locally
Sympathetic Regulation of Vessels
BP = CO x TPR
BP= blood pressure, CO = cardiac output, TPR = total peripheral resistance
WIDESPREAD arteriolar vasoconstriction → increase Total Peripheral Resistance (TPR) → increase preasure (Q = P/R) → increase blood flow
decrease in BP → key stimulus to activate SNS
most arterioles are richly innervated with sympathetic nerve fibers
norepinephrine will stimulate arteriolar VASOCONSTRICTION in most organs
sympathetic nervous system activation → most arteries constrict → increase total peripheral resistance → increase pressure
automatic tone = background of sympathetic activity
decrease in sympathetic activation → vasodilation → decrease in resistance → decrease pressure
increase in sympathetic activation → vasoconstriction → increase in resistance → increase in pressure → increase blood flow
important exceptions: cerebral vessels, coronary blood vessels, pulmonary vasculature
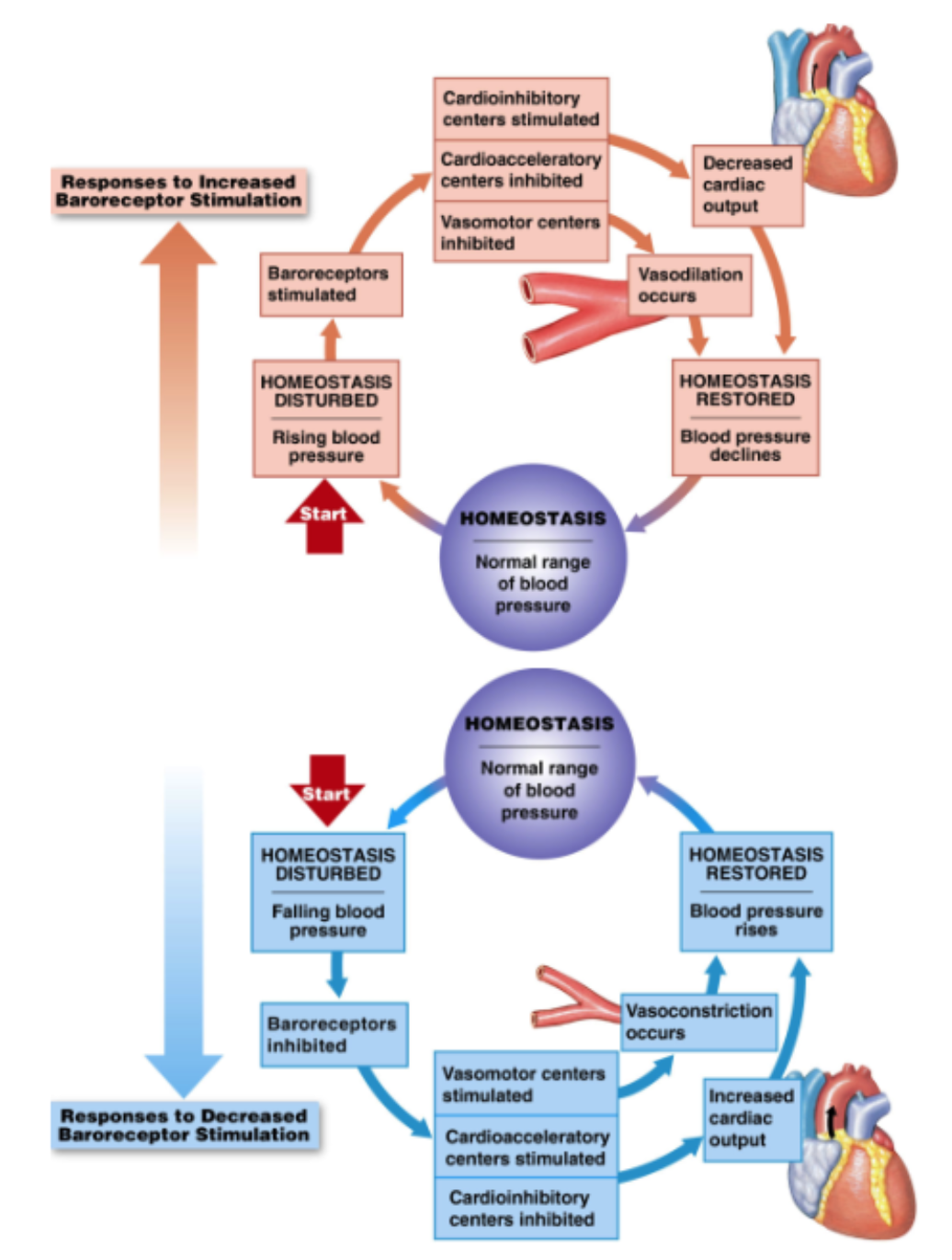
Baroreceptors: definition and location
Baroreceptors: respond to changes in stretch
increased stretch → increased firing of sensory nerves
decreased stretch → decreased firing of sensory nerves
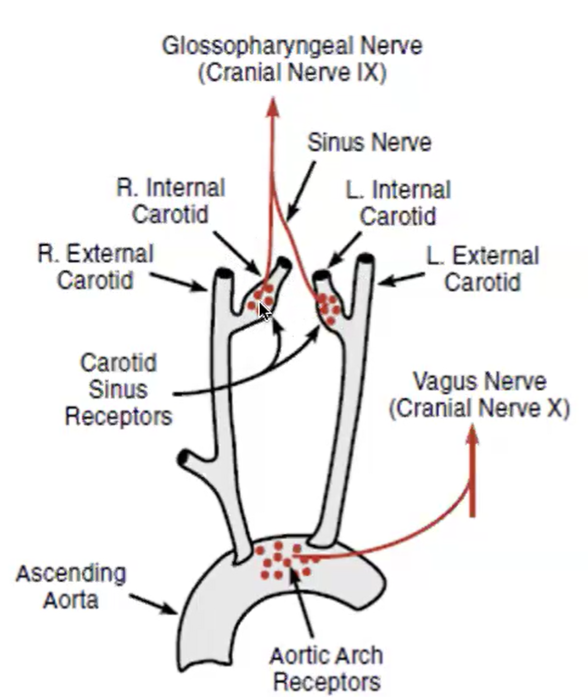
located at 2 major sites
1. wall of aortic arch → sensory nerve = vagus nerve
2. carotid sinus → sensory nerve = glossopharyngeal nerve
all sensory input going to the medulla oblongata
Baroreceptor reflex feedback loop
detectors = baroreceptors in carotid sinus and aortic arch
afferent pathways (sensory info → brain) = Cranial nerve IX (glossopharyngeal) for carotid sinus and X (Vagus) for aortic arch
integration center = medulla: cardiovascular control center
cardioinhibitory/ cardioacceleratory centers
vasomotor centers
efferent pathways (brain to effector organ) = sympathetic and parasympathetic nerves
Effector organs:
heart (conduction system and myocytes)
cardiac output
blood vessels (vascular smooth muscle)
vasodilation/vasoconstriction
BP increased → more stretch in baroreceptors → increase signal → send info back via cranial nerve → medulla oblongata: vasomotor center inhibited (dilate vessel), cardioacceleratory center inhibited, cardioinhibatory center stimulated (slow down heart rate decrease cardiac output) → stimulate PNS → reduced BP
BP decreased → less stretch in baroreceptors → decreased signal → info travel up cranial nerve → Medulla: vasomotor center stimulated (vasoconstriction), Cardioacceleratory center stimulated (speed up heartrate → increase cardiac output), cardioinhibitory center inhibited, → stimulate SNS → increased BP
Chemoreceptor Reflexes
Specialized receptors
respond to pH levels in blood
elevated CO2 → decrease in pH (CO2 = acidic)
→ increase blood flow → increase gas exchange with lungs
respond to O2 levels in blood
increase blood flow through lungs to bring more O2 in
Receptors in sensory neurons of carotid sinus and aortic arch
decrease pH and decrease in O2 → (want to increase blood fllow and blood pressure) → medulla: vasomotor centers stimulated (vasoconstriction), cardioacceleratory centers stimulated (increase CO), cardioinhibitory centers inhibited → increase BP and Q
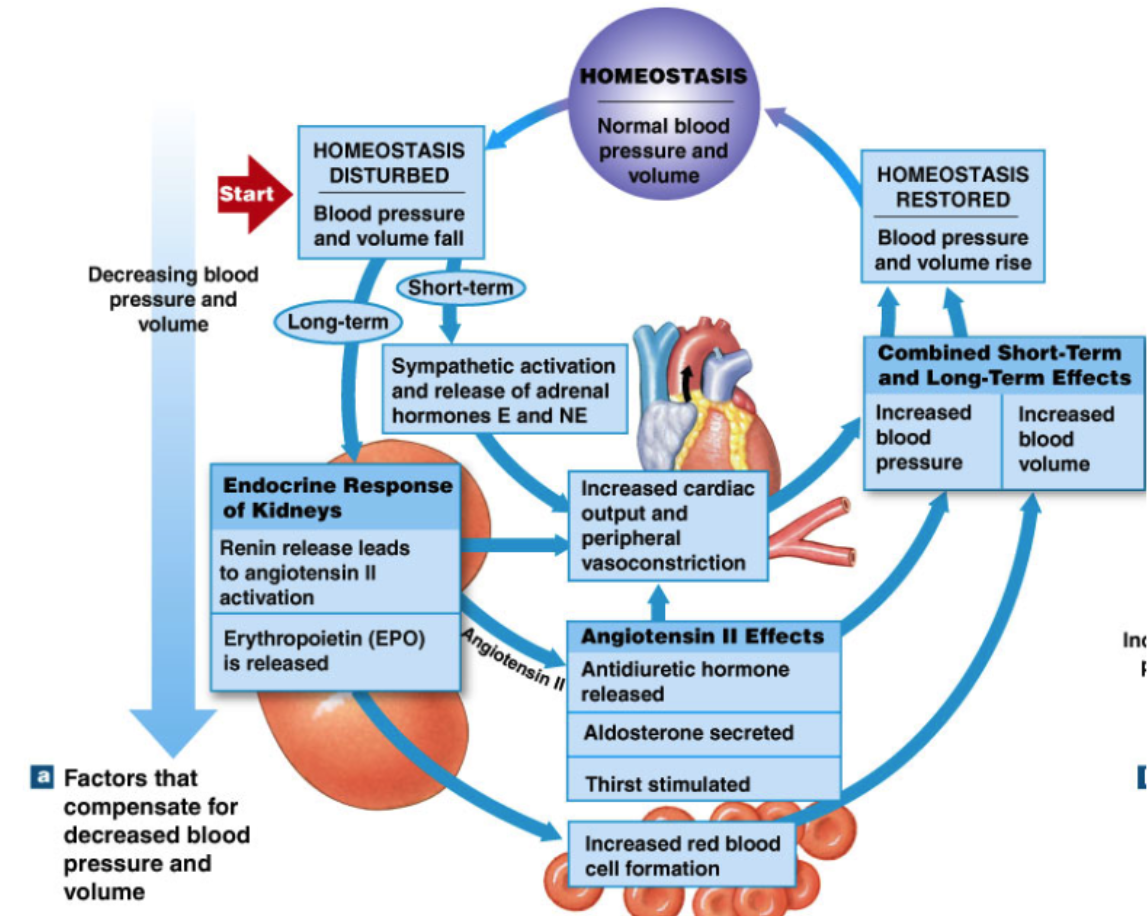
Renal Regulation of Blood Pressure: RAAS (IMPORTANT!!!) (increase BP)
Renin-Angiotensin-aldosterone system
long term effect
kidneys release RENIN (hormone) in response to
decrease in BP (stretch receptors in kidney tubules → renal baroreceptors)
sympathetic activation (kidney tubules innervated by sympathetic nerves)
decreased flow of sodium through kidney tubules
in blood, RENIN converts ANGIOTENSINOGEN → Angiotensin 1 (prohormone)
in lung, ACE enzyme converts Angiotensin 1 → angiotensin 2
Effector organs of angiotensin 2 (ANG II) → more effective than norepinephrine for vasoconstriction
Blood vessels → vasoconstriction (increase TPR→ total peripheral resistance)
Heart → increased cardiac output
Adrenal Cortex → aldosterone release
increase Na+ reabsorption by kidney → reabsorb water → increase blood volume → increase BP
Hypothalamus
ADH release → increase water reabsorption by kidney → increase blood volume → increase BP
stimulate thirst
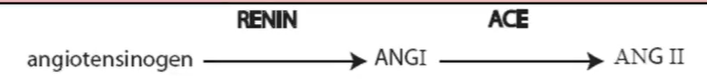
RAAS feedback loop
Kidney sensitive to BP decrease
→ increase release of RENIN
→ Renin converts Angiotensinogen → Angiotensin 1
→ in lungs ACE converts Angiotensin 1 → Angiotensin 2
→ Ang II affects all the stuff:
Blood pressure → vasoconstriction
heart → increased CO
adrenal cortex → aldosterone release
hypothalamus → ADH release and increased thirst
→ kidneys also increase red blood cell formation
→ increase oxygen carrying capability
Natriuretic Peptides (decrease BP)
ANP → atrial natriuretic peptides
Detectors: baroreceptors in walls of right atrium
hormonal response to atrial stretch:
when atrium stretch → atrial myocytes release ANP (atrial natriuretic peptide)
effects of ANP → decrease BP
vasodilation
increased sodium and water excretion
decrease blood volume → decrease BP
block: ADH, aldosterone, norepinephrine
block all the effects
increased blood in atria → increased atrial stretch → increased ANP release → increased sodium and water excretion → decreased blood volume → decreased BP
Chemoreceptor reflexes
detect O2 and pH in aortic arch are carotid sinus
CO2 + H2O → H2CO3 → H+ + HCO3-
special circulations
coronary circulations
supply heart with blood
normally 60ml/minute/100g of heqrt
w/ exercise > 250mL/minute/100g of heart
max blood flow = coronary reserve
ATP metabolites → vasodilation
Regulation of coronary blood flow
coronary circulation = blood supply to heart.
L and R coronary arteries take blood to heart
at rest: blood flow through coronary arteries → 60mL/min per 100g tissue (dont need to remember)
exercise: increases to >250 mL/min per 100 g tissue → “coronary reserve”
increase blood flow to heart (local)
vasodilate artery → reduce resistance (resistance more important for local) → increase blood flow
breakdown products (metabolites) of ATP production → vasodilation of coronary arteries
heart uses ATP → break down ATP into metabolites → metabolites serve as potent vasodilators
SNS → release epinephrine from adrenal gland → causes vasodilation of coronary arteries (opposite of most vessels)
increased work of heart → higher rate of ATP breakdown → increased vasodilation → increased epinephrine → further vasodilation → increased coronary blood flow
Angina = coronary spasms which temporarily block blood flow
Myocardial Infarction (heart attack) = total blockage of part of coronary circulation → death of cardiomyocytes
left coronary artery is most commonly blocked → descending into anterior ventricular septum
Cerebral Circulation
autoregulation → brain is least tolerant to ischemia (lack of blood flow)
brain dependent on oxygen and glucose
death of cells within minutes of ischemia
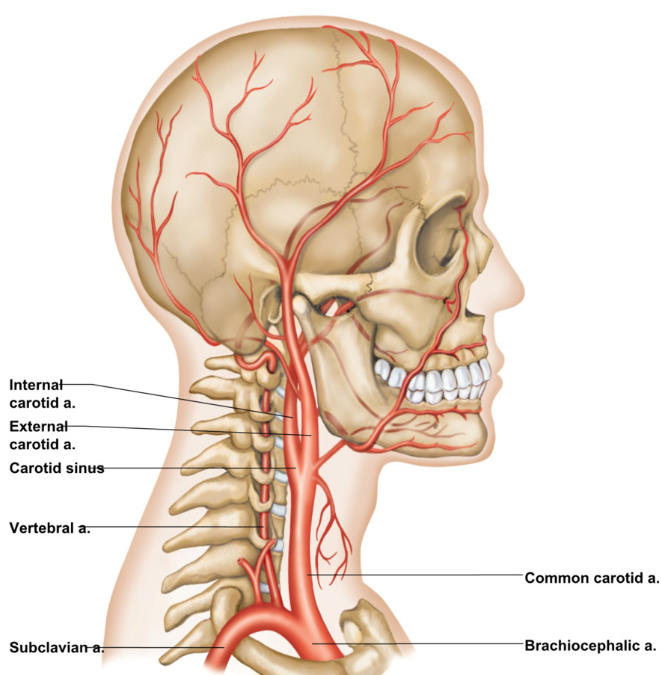
Brain receives blood through 4 source arteries
2 internal carotid arteries (branch off common carotid)
external → blood outside of skull
internal → blood to brain via carotid canal
2 vertebral arteries
blood supplies 15% of resting cardiac output to the brain
brain is least tolerant organ to ischemia (reduction of blood flow)
cell death within minutes
Cranial cavity is fixed space → limits volumetric changes
too much blood flow → hemorrhage → extra pressure against brain damages neurons
Autoregulation of Cerebral Blood Flow
Blood flow to brain is maintained at constant level over wide range of pressures (mean arterial pressure) → constant brain blood flow
Autoregulation:
prevents increase in blood flow and intracranial pressure when blood pressure increases
maintains adequate blood flow when blood pressure decreases
Process: vasoconstriction/vasodilation as needed
metabolic mechanisms (increases in demand or waste→ act as metabolic vasodilators)
myogenic mechanism (increased stretch in smooth muscle of of arterioles → contracts in response)
autoregulatory range = 50 -150 mmHg
Splanchnic Circulation
blood flow through GI tract: stomach, intestines, pancreas, spleen, liver
hepatic portal system:
substances absorbed in GI tract travel first to liver → detoxify before going to heart
Splanchnic circulation can serve as blood reservoir
receives 25% of resting cardiac output
mobilize blood from splanchnic circulation when needed (like in fight or flight) → redirect
splanchnic circulation heavily regulated by autonomic nervous system
increased SNS activity → vasoconstriction
less output to splanchnic → to SNS
hormonal and local metabolite regulate blood flow with change activity of gi tract
Characteristics of Blood
homogenous connective tissue
Blood:
temperature = 100 F (higher than body temp → transfers heat)
pH = 7.35 - 7.45
viscous: more viscous than water due to solid elements
average blood volume = 5 Liters
Components of blood:
Plasma: Extracellular fluid
Formed Elements: blood cells
red blood cells
white blood cells
platelets
serum → fluid left after natural clotting
plasma → fluid left without clotting
hematocrit → % of RBC
Plasma
ECM of blood
91% water
Proteins (7% of weight)
Albumins:
regulate osmotic pressure → creates osmotic gradient to draw water
transport steroid hormones → act as carrier proteins sometimes
buffer
Globulins (round protein)
immunity → produced by immune cells
transport steroid hormones and thyroid hormone → carrier protein
Fibrinogen
facilitates blood clotting
serum = plasma without clotting factor → no fibrinogen
Other solutes (2%):
ions
sodium, potassium, calcium, magnesium, chloride, iron, phosphate, hydrogen, hydroxide, bicarbonate
Nutrients: glucose, amino acids, triglycerides, cholesterol, vitamins
Waste products
protein breakdown → urea, uric acid, creatine
RBC breakdown → bilirubin
lactic acid → product of anaerobic respiration
Gases: O2, CO2, N2
regulatory substances: enzymes and hormones
Formed Elements
Erythrocytes (RBCs) (99.9%) → oxygen and CO2 transport
Leukocytes (WBCs) (<0.1%) → immune responses
Thrombocytes (platelet) → clotting
Red Blood Cells (erythrocytes)
Function: transport of gases (oxygen and carbon dioxide throughout body)
Numbers (affected by testosterone)
Females (4.2 - 5.5 million per uL)
usually menstruate → lower blood
Males (4.5 -6.3 million per uL)
testosterone → more RBC
Shape/Function relationship
lack organelles
biconcave disc shape
have bunch of protein → hemoglobin (95% of RBC)
flat →
higher surface area to volume ratio
function of RBC → diffusion of oxygen → easier to diffuse over large SA
stackable → fit through capillary beds (almost same size)
Lifespan:
120 days
no organelles → no repair mechanism
constantly move → damage easily
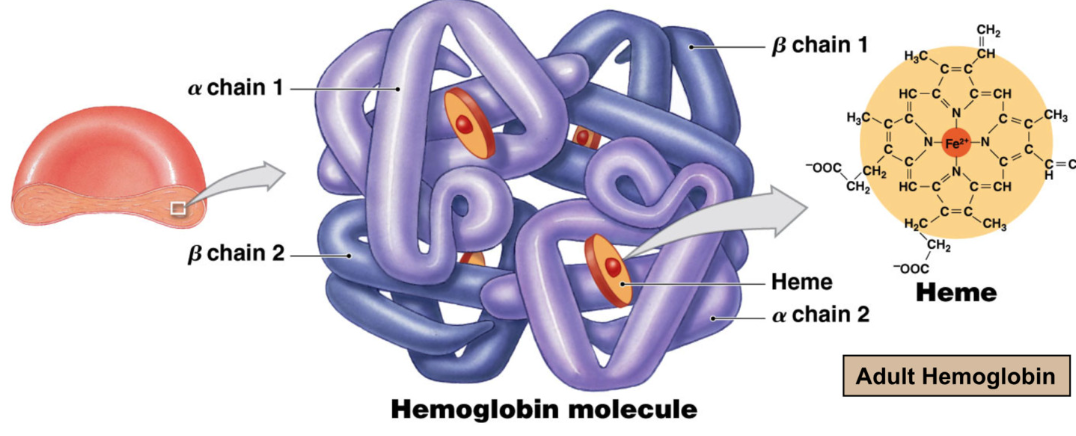
Hemoglobin
Function: Gas transport → majority of oxygen in blood is bound to hemoglobin
hemoglobin quaternary structure (multiple proteins) made of
4 hemes and 4 globin proteins
each globin gets 1 heme
2 alpha chains
2 beta chains
heme
each RBS contains 280 million hemoglobin molecules → carry 1 billion oxygen molecules
Heme = iron containing pigment complex
oxygen reversibly binds to iron in heme
Oxyhemoglobin → oxygen bound to hemoglobin (bright red)
Deoxyhemoglobin → oxygen removed from hemoglobin (darker red)
Life Cycle of Red Blood Cells
120 days
synthesized in red bone marrow in epiphysis of long bone and in flat bone via erythropoiesis
body constantly monitors RBC → don’t want to lose RBC contents to hemolysis (RBC exploding)
RBCs constantly degraded and replaced → macrophages in sinusoidal capillaries monitor RBC in spleen, liver, lymph nodes
if macrophages see RBC is at end of life → eat and recycle materials
globin broken down into amino acids and recycled
Heme Breakdown:
iron recycled
heme without iron converted → Biliverdin (green)
Biliverdin converted → Bilirubin (yellow/orange)
bilirubin excreted in bile to large intestine
bacteria break bilirubin → stercobilin → urobilin
stercobilin brown → poop is brown yippee
urobilin reabsorbed from digestive system → blood stream → kidney → pee
jaundice: accumulation of bilirubin causing yellowing of skin and eyes
The Spleen
5 inch organ in upper left of abdominal cavity
sinusoidal capillary beds allow for free exchange of blood cells
red pulp: filtration of RBC → red blood cells and macrophages for RBC monitoring and recycling
white pulp: lymphoid tissue housing T and B lymphocytes
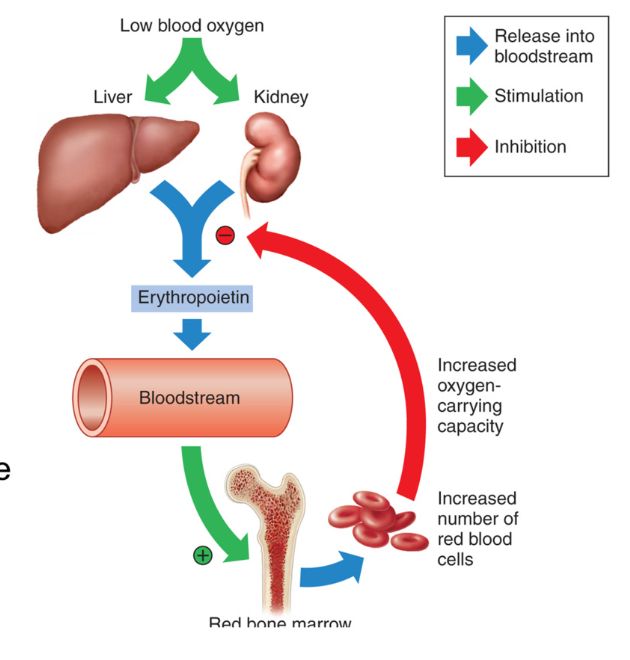
Regulation of Erythropoiesis (RBC synthesis)
synthesis of erythrocytes (RBC) regulated by hormone Erythropoietin (EPO)
excreted by Kidneys
EPO released when O2 levels are low → stimulates production of RBC → more RBC to carry oxygen → increases carrying capacity of oxygen
Testosterone release more EPO
Signal for release of erythropoietin (EPO)
HYPOXIA = low oxygen
androgens
Hematocrit
Percent volume of RBC in blood
normal range → 40-50%
55% plasma
buffy coat (WBC and platelets)
45% RBC hematocrit
Elevated Hematocrit from:
increasing RBC
decreasing plasma
Decreased hematocrit
low RBC
excess plasma