Biochem Concepts
1/150
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
151 Terms
Non-protein Cofactor
A non-protein molecule is a broad category that includes any non-protein molecule or ion that assists an enzyme. This includes inorganic cofactors like magnesium, iron, zinc, or other metals. It also includes organic cofactors like NAD+ or FAD used in redox reactions, or coenzyme A, and ATP.
Apoenzyme
The protein part of an enzyme that is inactive on its own because it lacks the necessary cofactor (which can be a non-protein molecule OR ion) It requires the cofactor to become an active enzyme. When it binds with its cofactor, it becomes a holoenzyme.
Holoenzyme
An enzyme that needs a nonprotein cofactor. It is an active enzyme made of the apoenzyme plus its cofactor(s)
Co-substrate
Transiently associated with an enzyme. They bind temporarily, participate in reaction, and then are released. They are chemically altered but are regenerated in subsequent reactions.
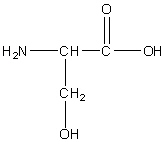
Serine
Ser, S
R group: a methylene group connecting the central carbon to the hydroxyl group
Polar uncharged
Involved in hydrogen bonding due to hydroxyl group
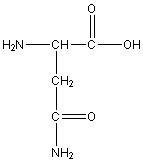
Asparagine
Asn, N
R group: side chain amide group (-CH2-CONH2)
Polar uncharged
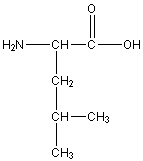
Leucine
Leu, L
R group: -CH2-CH(CH3)2
Uncharged non-polar
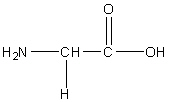
Glycine
Gly, G
R group: -H. Just a single hydrogen
Uncharged non-polar
The smallest and only achiral amino acid. Involved in the synthesis of collagen.
Considered the “helix breaker”
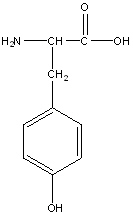
Tyrosine
Tyr, Y
R group: aromatic ring with an alcohol at the end
Uncharged polar amino acid due to the presence of hydroxyl on its aromatic ring. This group allows hydrogen bonds to form with water molecules
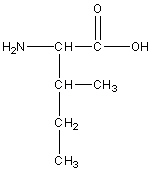
Isoleucine
Ile, I
R group: amine and carboxyl group
Non-polar neutral
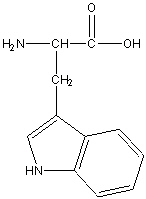
Tryptophan
Tyr, Y
R group: two fused aromatic rings with a nitrogen atom
Aromatic Non-polar, hydrophobic amino Acid
The large aromatic índole ring can contribute significantly to protein folding and stability through interactions with other amino acids
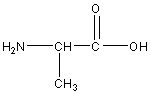
Alanine
Ala, A
R group: -CH3 (methyl group)
Nonpolar uncharged amino acid
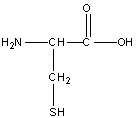
Cysteine
Cys, C
R group: -CH2-SH (Methylene and sulfhydryl group)
Neutral Polar amino
-SH group can ionize to form negatively charged thiolate ion (S-) under certain conditions
Plays a critical role in protein structure by forming disulfide bonds with cysteine residues, contributing to stabilizing protein folding and maintaining tertiary structure
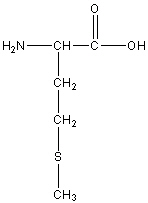
Methionine
Met, M
R group: -CH2-CH2-S-CH3
Neutral Non-polar
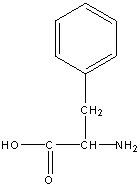
Phenylalanine
Phe, F
R group: a benzene ring connected to a methylene
Neutral non-polar
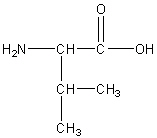
Valine
Val, V
Neutral non-polar
R group: isopropyl group (-CH(CH3)2)
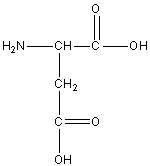
Aspartic Acid or Aspartate
Asp, D
Negatively Charged Polar
R group is -CH2COOH
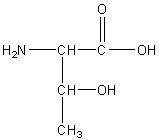
Threonine
The, T
Neutral Polar
R group: hydroxyl group attached to a carbon that is also attached to a methyl group.
Hydroxyl group allows it to form hydrogen bonds
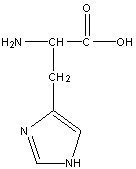
Histidine
His, H
R group: 5 sided aromatic ring with two nitrogen atoms
Uncharged polar amino acid
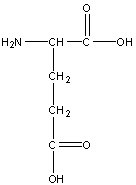
Glutamic acid or glutamate
Glu, E
R group: -CH2-CH2-COOH
Negatively charged polar
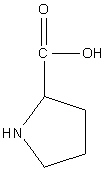
Proline
Pro, P
Neutral non-polar
R group: 5 sided ring with one nitrogen atom
Found in a bend of proteins
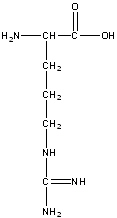
Arginine
Arg, R
Polar positive
R group is 3 methyl groups (-CH2-CH2-CH2) followed by (-NH-C(=NH)-NH2) at the end
It’s positive charge helps it interact with negatively charged molecules such as DNA and RNA, and is often found in the binding sites of proteins that bind with nucleic acids
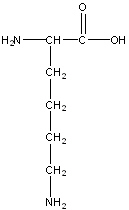
Lysine
Lys, K
Positive Polar
R-group: -(CH2)4-NH2
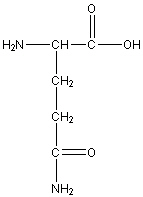
Glutamine
Gln, Q
Neutral Polar
R group: -CH2-CH2-C(=0)-NH2
How does pH relate to H+ ion concentration?
A lower pH means a higher concentration of H+ ions (acidic) while a higher pH means a lower concentration of H+ ions (basic)
What is pKa, and how does it relate to an amino acid’s ionizable side chain?
pKa is the pH at which 50% of the ionizable group is protonated. If the pH is below the pKa, the group tends be protanated (gains H+); if above, it tends to be deprotonated (loses H+)
which amino acids have ionizable side chains, and what are their typical charges at physiological pH?
Ionizable side chains include acidic groups (aspartic and glutamic acids, that are typically negative at physiological pH) and basic groups (lysine, arginine, histidine, typically positive at physiological pH)
How does protonation or deprotonation affect the charge of ionizable side chains?
Protonation (gain of H+) usually adds a positive charge, while deprotonation (loss of H+) removes a positive charge or adds a negative one, depending on the group.
What factors commonly affect enzyme activity, and how?
Temperature, pH, and substrate concentration affect enzyme activity by influencing reaction rates, enzyme structure, or active site affinity.
These factors impact enzyme shape and the energy needed to reach the transition state
Gibbs free energy (delta G) remains UNAFFECTED by enzymes.
What happens to enzyme activity as temperature increases, and why?
Enzyme activity increases with temp due to higher kinetic energy, leading to more frequency collisions between enzymes and substrates.
If the temperature increases beyond the optimal temperature, denaturation occurs, causing a loss of structure and function
How does pH affect enzyme activity, and what happens outside the optimal pH range?
Each enzyme has an optimal pH for maximum activity. Deviations in pH can disrupt the ionization of active site residues, altering enzyme shape and reducing activity, potentially leading to denaturation at extreme pH levels.
How does substrate concentration influence enzyme activity?
Increasing substrate concentration boosts enzyme activity until saturation is reached. Beyond saturation, all active sites are occupied, and adding more substrate does not increase the reaction rate.
How does the structure of an enzyme relate to its function?
An enzyme’s specific 3D shape allows its active site to bind substrates with high specificity. Factors like temperature and pH can alter this shape, affecting enzyme function. The primary, secondary, tertiary, and quaternary structures are crucial for enzyme activity.
What does Gibbs free energy (ΔG) indicate in a biochemical reaction?
ΔG indicates the spontaneity of a reaction; a negative ΔG means the reaction can proceed spontaneously. Enzymes lower the activation energy (Ea) but do not affect ΔG.
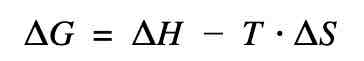
What does each coefficient of this equation mean?
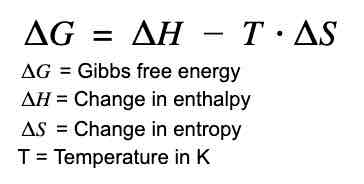
What is the difference between activation energy (Ea) and Gibbs free energy (ΔG) in enzymatic reactions?
Activation energy (Ea) is the energy required to start a reaction, and enzymes lower Ea. Gibbs free energy (ΔG) represents the energy difference between reactants and products, indicating spontaneity; enzymes affect Ea but not ΔG.
What are “optimal conditions” for enzymes, and why are they important?
Optimal conditions refer to specific temperature and pH ranges where an enzyme functions best. Maintaining these conditions is crucial for enzyme efficiency and preventing denaturation.
What is the Michaelis constant (Km) and what does it indicate about enzyme activity?
Km is the substrate concentration at which an enzyme works at half its maximum rate (Vmax/2). A low Km indicates high affinity between enzyme and substrate, while a high Km suggests lower affinity.
Which amino acid is attached to the heme ring of hemoglobin (Hb)?
Histidine is the amino acid that coordinates with the iron atom in the heme group of hemoglobin, playing a crucial role in oxygen binding and release.
What is the structural composition of hemoglobin?
Hemoglobin is a tetramer composed of four polypeptide chains: two alpha (α) and two beta (β) chains, each containing a heme group that binds oxygen. Histidine residues are important for stabilizing the heme’s interaction with iron.
What is the function of the heme group in hemoglobin?
The heme group binds oxygen in a reversible manner due to the iron ion (Fe²⁺) at its center. The conformational changes in hemoglobin upon oxygen binding enhance its affinity for additional oxygen molecules.
What role does histidine play in hemoglobin’s function?
What is the Bohr effect, and how does it facilitate oxygen unloading in tissues?
The Bohr effect describes how increased CO2 and lowered pH (more H+ ions) stabilize the T state, promoting oxygen release. In active tissues, high CO2 and acidic pH shift the curve right, enhancing oxygen unloading.
What causes a rightward shift in the hemoglobin dissociation curve, and what is the physiological significance?
A rightward shift occurs with increased CO2, higher temperature, and lower pH. This shift reduces hemoglobin’s oxygen affinity, facilitating oxygen release to tissues in need.
What factors lead to a leftward shift in the hemoglobin dissociation curve, and how does this effect oxygen affinity?
A leftward shift occurs with decreased CO2, lower temperature, and higher pH. This increases oxygen affinity, favoring oxygen loading in the lungs.
How does smoking affect the partial pressure of oxygen (pO2) and hemoglobin’s oxygen saturation?
Smoking increases carbon monoxide (CO), which binds to hemoglobin with high affinity, reducing available oxygen binding sites. This lowers oxygen saturation, impairing oxygen delivery to tissues.
How does CO2 concentration influence hemoglobin’s affinity for oxygen?
Increased CO2 lowers blood pH, shifting hemoglobin to the T state (right shift), promoting oxygen release. Lower CO2 increases pH, stabilizing the R state (left shift), promoting oxygen binding.
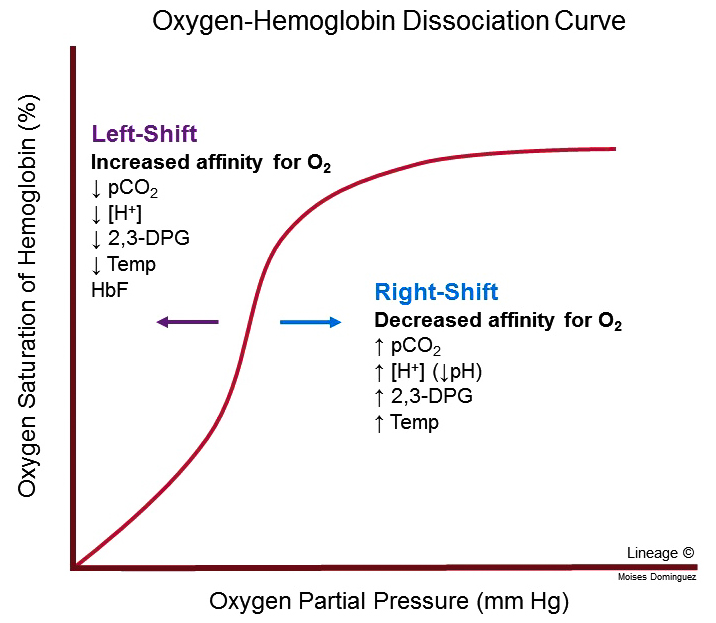
How does the steep portion of the hemoglobin dissociation curve influence oxygen release in tissues?
The steep portion of the hemoglobin curve reflects cooperative binding, where a small drop in pO2 leads to a large release of oxygen. This property allows hemoglobin to release oxygen efficiently in tissues with lower pO2, such as active muscles.
How does exercise affect the oxygen dissociation curve and oxygen delivery to tissues?
Exercise increases CO2 production and temperature, shifting the curve right. This rightward shift enhances oxygen offloading in active muscles where it’s needed most.
How does oxygen binding affinity differ between hemoglobin (Hb) and myoglobin (Mb)?
Hemoglobin (Hb) has a lower binding affinity for oxygen than myoglobin (Mb) and shows cooperative binding, which means its affinity changes depending on pO2. Myoglobin has a higher, more constant affinity for oxygen, making it suitable for oxygen storage in muscle tissues.
How does the partial pressure of oxygen (pO2) influence oxygen binding to hemoglobin (Hb)?
Hemoglobin’s affinity for oxygen increases as pO2 increases, displaying a sigmoidal binding curve. This cooperative binding allows Hb to pick up oxygen in the lungs (high pO2) and release it in tissues (low pO2).
Why does myoglobin (Mb) have a hyperbolic oxygen-binding curve rather than a sigmoidal one?
Myoglobin has a single binding site for oxygen, so its affinity for O2 does not change with pO2. This results in a hyperbolic curve, showing a constant, high affinity for oxygen that is not influenced by cooperative binding.
What does it mean that hemoglobin’s oxygen-binding curve is sigmoidal?
The sigmoidal shape indicates cooperative binding, where each oxygen molecule bound increases Hb’s affinity for the next. This allows Hb to load oxygen efficiently at high pO2 (in lungs) and release it where pO2 is lower (in tissues).
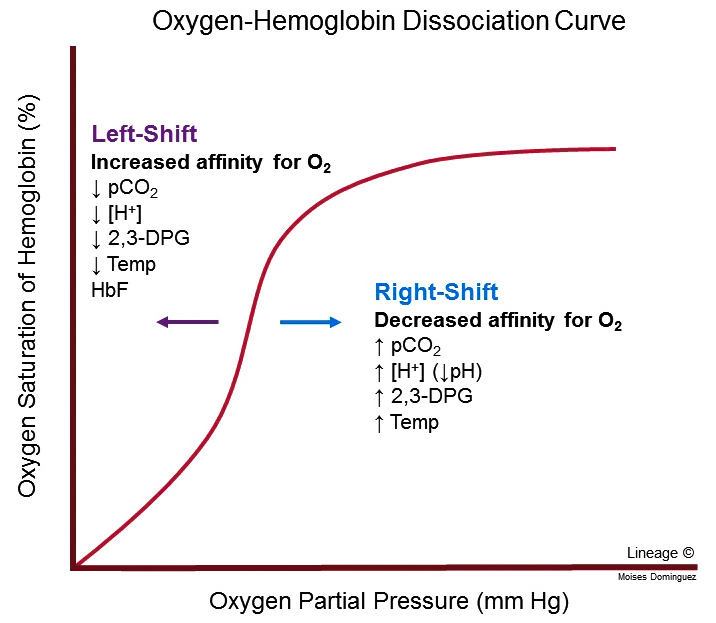
How is myoglobin’s role in muscle tissue different from hemoglobin’s role in the bloodstream in terms of oxygen affinity?
Myoglobin acts as an oxygen storage molecule in muscles due to its high affinity and less sensitivity to pO2 changes, releasing oxygen only when tissue pO2 is very low. Hemoglobin, with variable affinity, transports oxygen in the blood and releases it in tissues more readily due to cooperative binding.
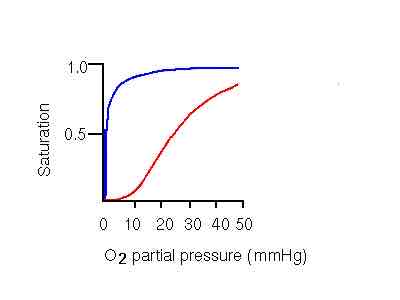
Which line is refers to myoglobin, and which one refers to hemoglobin
Blue: myoglobin, red: hemoglobin
Hydrolase
Function: Catalyzes the cleavage of bonds (e.g., peptide, ester, glycosidic) by adding water.
ATP Use: Does not use ATP; relies on water to break bonds.
Examples: Protease (breaks peptide bonds), Glycosidase (breaks glycosidic bonds).
Oxidoreductase
Function: Catalyzes oxidation-reduction (redox) reactions, where one molecule is oxidized and another is reduced.
ATP Use: Does not directly use ATP, but may rely on cofactors like NAD⁺, NADP⁺, or FAD for electron transfer.
NAD+ serves as a coenzyme oxidizing agent
Examples: Dehydrogenase, Oxidase.
Transferase
Function: Transfers functional groups (e.g., methyl, phosphate, amino) from one molecule to another.
ATP Use: Often uses ATP, especially in kinases that transfer phosphate groups.
Examples: Kinase (transfers phosphate groups from ATP), Transaminase (transfers amino groups without ATP).
Isomerase
Function: Catalyzes the rearrangement of atoms within a molecule, converting it to its isomer.
ATP Use: Does not use ATP directly.
Examples: Phosphoglucose isomerase (converts glucose-6-phosphate to fructose-6-phosphate).
Ligase
Function: Joins two molecules together, typically requiring ATP.
ATP Use: Requires ATP for bond formation, as in DNA synthesis or other biosynthetic processes.
Examples: DNA ligase (joins DNA strands), Synthetase.
Translocase
Function: Moves ions or molecules across membranes or within cellular compartments.
ATP Use: Often uses ATP or other forms of energy (e.g., electrochemical gradients) for transport.
Examples: ATP synthase (uses proton gradient to synthesize ATP), Sodium-potassium pump (uses ATP to move ions across cell membranes).
What is the Gibbs Free Energy Equation?
ΔG° = -RT ln(K), where ΔG° is the standard Gibbs free energy change, R is the universal gas constant, T is the temperature in Kelvin, and K is the equilibrium constant.
What does ΔG° represent in thermodynamics?
Standard Gibbs free energy change, indicating the spontaneity of a reaction under standard conditions (1 M concentration, 1 atm pressure, and a specified temperature).
If Keq=0, what can be said about ΔG°?
ΔG° = 0, indicating that the reaction is at equilibrium, and neither reactants nor products are favored.
What does a negative ΔG° value indicate about a reaction?
The reaction is spontaneous in the forward direction, meaning products are favored at equilibrium.
What does a positive ΔG° value indicate about a reaction?
The reaction is non-spontaneous in the forward direction, meaning reactants are favored at equilibrium.
What does the Law of Mass Action state?
The rate of a chemical reaction is proportional to the product of the concentrations of the reactants, each raised to a power corresponding to their coefficients in the balanced equation.
How does the Law of Mass Action relate to Keq?
At equilibrium, the concentrations of reactants and products are constant, and the ratio of these concentrations is defined by Keq
What is the Michaelis-Menten equation?
Vo = vmax*[S]/Km+[S]
What does Vo represent in enzyme kinetics?
The initial velocity of the enzymatic reaction, measured at the start when substrate is added and before the reaction reaches equilibrium
What is Vmax?
The maximum velocity of the enzyme-catalyzed reaction, occurring when the enzyme is fully saturated with substrate.
What does Km represent?
The substrate concentration at which Vo is half of Vmax. It provides indication of the affinity of the enzyme for its substrate. A low Km indicates high affinity.
What does it mean when [S] = Km?
When [S]=Km, Vo=1/2Vmax. This indicates that the enzyme is operating at half its maximum capacity.
What is [S]?
The molar concentration of the substrate (measured in moles per liter, M) that the enzyme acts upon.The concentration of the substrate directly affects the initial velocity (Vo) of the enzymatic reaction. As [S] increases, the reaction rate typically increases until the enzyme becomes saturated with substrate.
The Michaelis constant (Km) is a specific concentration of substrate at which the reaction velocity is half of its maximum value (Vmax) When [S] equals Km, the enzyme operates at 50% of its maximum capacity.
Define first-order kinetics in the context of enzyme reactions.
First-order kinetics occurs when the reaction rate is directly proportional to [S], typically observed when [S] is much lower than Km.
Define zero-order kinetics in the context of enzyme reactions.
Zero-order kinetics indicates that the enzyme is saturated with substrate, meaning all active sites are occupied, and further increases in [S] do not affect the reaction rate.
When [S] is much higher than Km. The reaction rate becomes constant and is limited by the maximum rate of the enzyme.
How can one experimentally determine if a reaction follows first-order or zero-order kinetics?
By plotting the reaction rate versus [S], one can assess linearity: a linear relationship indicates first-order kinetics, while a plateau indicates zero-order kinetics.
![<p>By plotting the reaction rate versus [S], one can assess linearity: a linear relationship indicates first-order kinetics, while a plateau indicates zero-order kinetics.</p>](https://knowt-user-attachments.s3.amazonaws.com/744ef3d6-af20-4f8e-8e3b-88f552f908de.jpg)
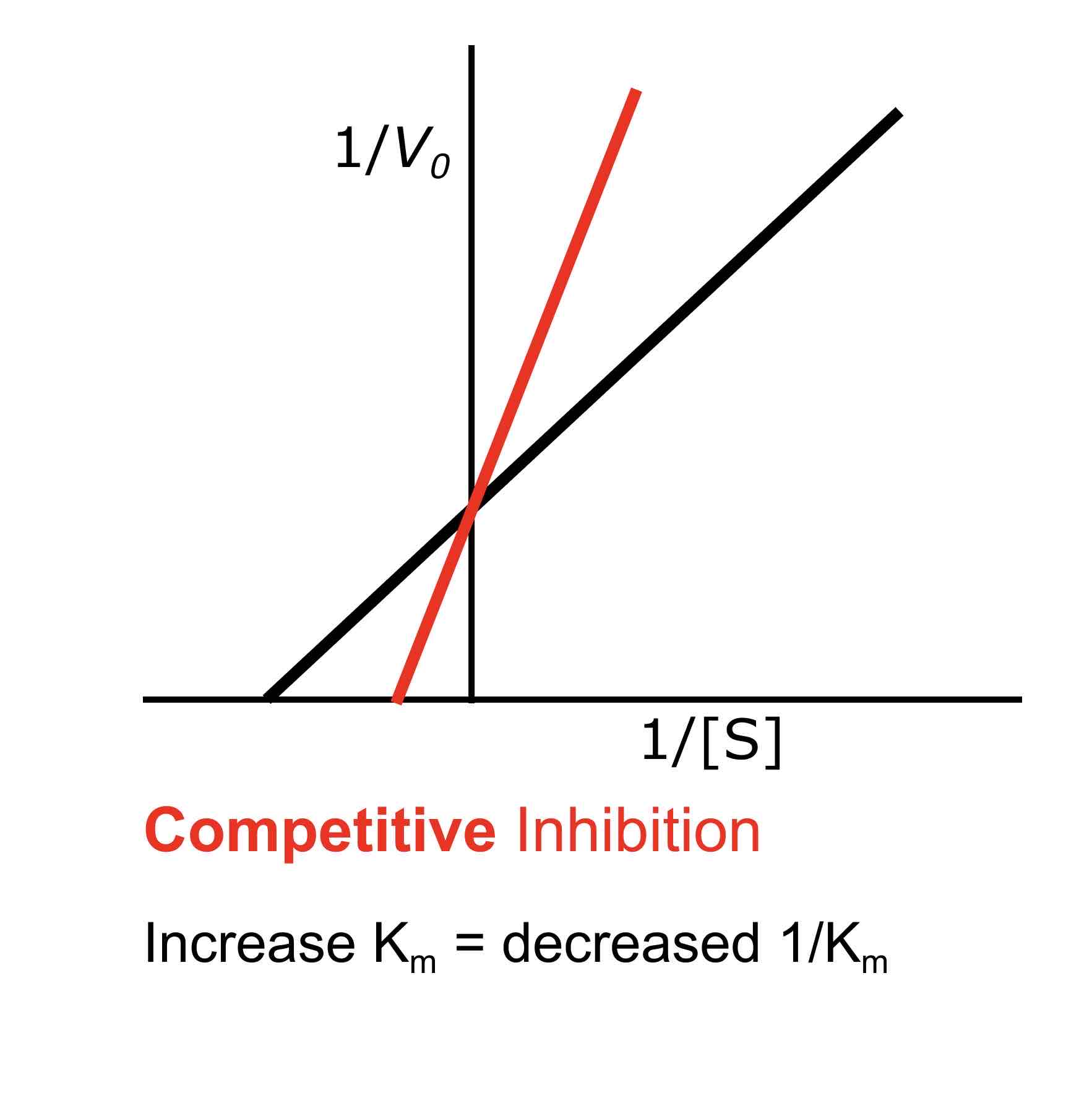
How does competitive inhibition affect Km?
Competitive inhibition increases Km, meaning that a higher substrate concentration is needed to reach half of Vmax. This occurs because the inhibitor competes with the substrate for the active site, decreasing the enzyme’s affinity for the substrate.
What is the effect of competitive inhibition on Vmax?
Competitive inhibition does not affect Vmax; it remains the same because the maximum reaction velocity can still be achieved if enough substrate is present to outcompete the inhibitor.
How does non-competitive inhibition affect Km
Non-competitive inhibition does not change Km, as the inhibitor does not prevent substrate binding but rather reduces the overall number of active enzyme sites available for reaction.
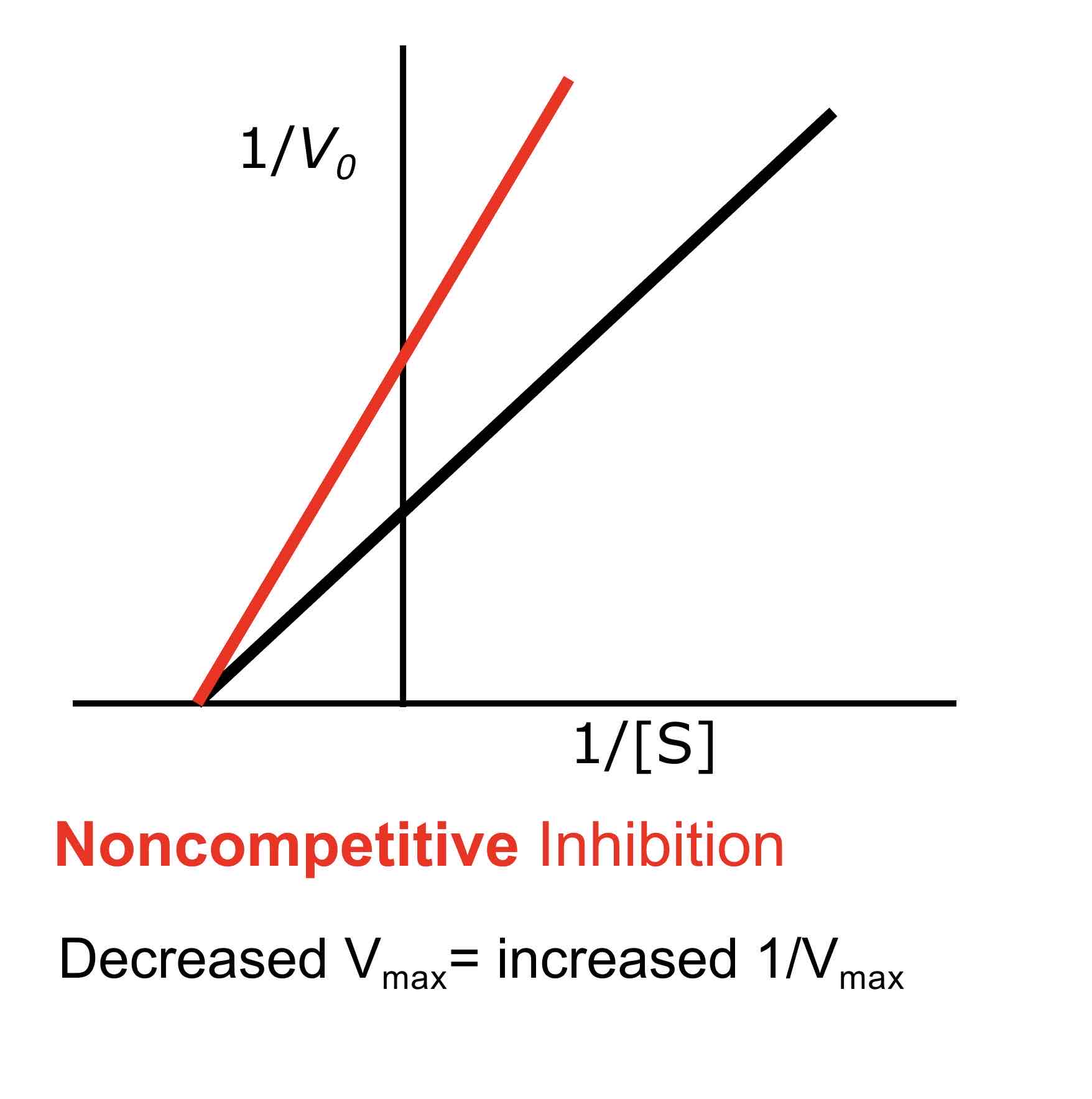
What is the effect of non-competitive inhibition on Vmax?
Non-competitive inhibition decreases Vmax because the maximum reaction velocity is reduced due to fewer active enzyme molecules being available for catalysis.
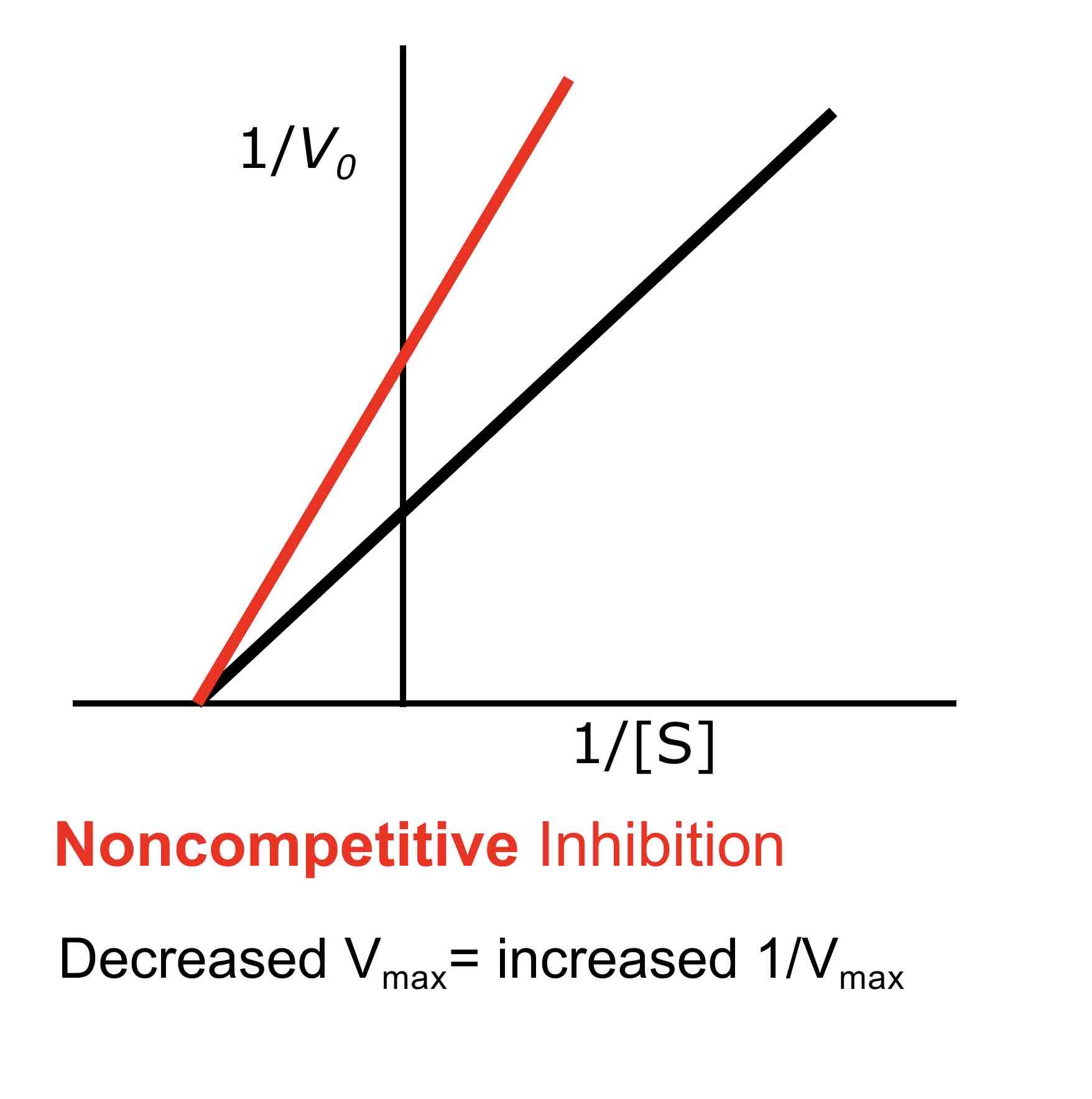
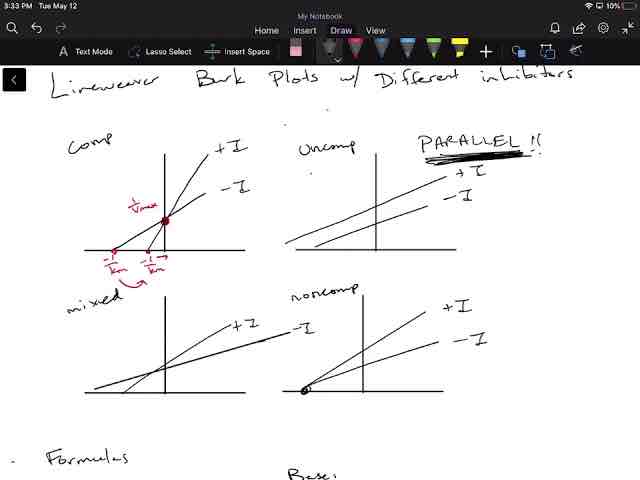
What is a Lineweaver-Burke plot?
A Lineweaver-Burke plot is a double reciprocal plot of 1/V0 (reaction velocity) versus 1/[S] (substrate concentration). It linearizes the Michaelis-Menten equation, allowing for the determination of Km and Vmax from the slope and intercepts of the line.
![<p><span>A Lineweaver-Burke plot is a double reciprocal plot of 1/V0 (reaction velocity) versus 1/[S] (substrate concentration). It linearizes the Michaelis-Menten equation, allowing for the determination of Km and Vmax from the slope and intercepts of the line.</span></p>](https://knowt-user-attachments.s3.amazonaws.com/9bb36357-4398-466a-8c33-07d0c6377bec.png)
How can you determine the type of inhibition using a Lineweaver-Burke plot?
In a Lineweaver-Burke plot, competitive inhibition is indicated by lines that intersect on the y-axis (same Vmax but different Km), while non-competitive inhibition is indicated by lines that intersect on the x-axis (same Km but different Vmax).
What are the main differences between proteins and carbohydrates like cellulose?
Proteins are made up of amino acids and perform various functions such as catalysis, transport, and signaling, while carbohydrates like cellulose are composed of sugar molecules and primarily serve structural or energy storage roles.
Which biomolecules are primarily involved in enzymatic functions?
Proteins are the primary biomolecules involved in enzymatic functions, as they are composed of amino acids that can fold into specific three-dimensional structures necessary for catalyzing biochemical reactions.
Which amino acid contains a hydroxyl (-OH) functional group in its R group?
Serine contains a hydroxyl (-OH) functional group in its R group, making it polar and capable of forming hydrogen bonds.
Which amino acid has a sulfur-containing side chain?
Cysteine has a sulfur-containing side chain, specifically a thiol (-SH) group, which can form disulfide bonds important for protein structure stabilization.
What is the significance of amino acids with acidic functional groups, such as aspartic acid?
Aspartic acid contains a carboxylic acid (-COOH) functional group in its R group, giving it a negative charge at physiological pH, which plays a crucial role in enzyme active sites and protein interactions.
How do the side chains of basic amino acids like lysine contribute to protein function?
Lysine has an amine (-NH2) functional group in its side chain, which can accept protons and carry a positive charge at physiological pH, playing a key role in enzyme active sites and protein interactions.
What amino acid contains an imidazole functional group in its R group?
Histidine contains an imidazole functional group in its side chain, which can be positively charged at physiological pH, making it important for enzyme catalysis and protein binding sites.
What effect does an increase in CO2 concentration have on hemoglobin’s affinity for oxygen?
An increase in CO2 concentration decreases hemoglobin’s affinity for oxygen, promoting the release of oxygen from hemoglobin to the tissues. The decreased affinity allows for more efficient oxygen unloading in metabolically active tissues that produce CO2, enhancing oxygen delivery where it is needed most.
What role does bicarbonate play in the transport of CO2 and its effect on oxygen binding?
CO2 is converted to bicarbonate (HCO3-) in red blood cells, which helps maintain blood pH. This conversion contributes to the Bohr effect, as the resulting hydrogen ions lower pH and reduce hemoglobin’s affinity for oxygen.
How does the presence of 2,3-bisphosphoglycerate (2,3-BPG) interact with the effects of CO2 on hemoglobin?
Increased levels of CO2 lead to increased production of 2,3-BPG, which further decreases hemoglobin’s affinity for oxygen, facilitating oxygen release during hypoxia or high metabolic activity.
What is the overall effect of elevated CO2 levels on oxygen delivery in the body?
Elevated CO2 levels enhance oxygen delivery to tissues by decreasing hemoglobin’s affinity for oxygen, promoting efficient unloading of oxygen where it is most needed for cellular respiration.
What intermolecular forces are involved in amino acid and protein interactions?
The intermolecular forces involved include ionic bonds, hydrogen bonds, hydrophobic interactions, and van der Waals forces.
Rate the intermolecular forces in protein interactions from weakest to strongest.
Van der Waals forces
Hydrophobic interactions
Hydrogen bonds
Ionic bonds
How do ionic bonds form between amino acids in proteins?
Ionic bonds form between positively charged side chains (e.g., lysine, arginine) and negatively charged side chains (e.g., aspartate, glutamate), stabilizing the protein structure through electrostatic interactions.
What role do hydrogen bonds play in protein structure?
Hydrogen bonds occur between polar side chains (e.g., serine, threonine) or between backbone atoms, contributing to the secondary structure of proteins (e.g., alpha-helices and beta-sheets).
How do hydrophobic interactions influence protein folding?
Hydrophobic interactions drive the folding of proteins by causing nonpolar side chains (e.g., leucine, phenylalanine) to aggregate away from the aqueous environment, stabilizing the overall structure.
Why are van der Waals forces important in protein interactions?
Van der Waals forces, while weak, play a role in stabilizing protein structure by promoting close packing of atoms within proteins and contributing to the overall molecular interactions.