Chem1405 Final Exam Review Topics
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
How is the number 0.000430 expressed in scientific notation?
4.30 x 10^-4
What are the symbols for the following metric units: microgram, kilometer, milliliter, decisecond, and centiliter?
Microgram: µg, Kilometer: km, Milliliter: mL, Decisecond: ds, Centiliter: cL
What mass of NaOH is needed to produce 22.0 mL of 1.3 M solution?
Mass = Molarity x Volume = 1.3 mol/L x 0.022 L x 40 g/mol = 1.14 g NaOH.
What is the molarity of a solution made by dissolving 2.60 moles of KBr in 1030 mL of water?
Molarity = moles/volume(L) = 2.60 moles / 1.030 L = 2.52 M.
What volume of a solution with a density of 13.9 g/mL is needed to provide 311 grams of solution?
Volume = mass/density = 311 g / 13.9 g/mL = 22.36 mL.
How many microliters are in 533 quarts? (using conversion: 1 gallon = 3.79 L, 4 quarts = 1 gallon).
533 qts x 1 Gal/4 qts x 3.79 L/1 Gal x 1 µL/10^-6 L= 5.05 x10^8
Which test result is classified as a chemical property?
A) It reacts with water. B) It's density is 1.84 g/mL
C) It tastes sour. D) It is a white-colored solid
A) It reacts with base to form water.
How can a pure substance that cannot be separated by physical means and can be separated by chemical means be classified?
I. The species cannot be separated by physical means
II. The species can be separated by chemical means.
A) An element B) A Compound
C) A homogenous Mixture D) A heterogeneous mixture
B) a compound.
Which types of substances are considered pure substances?
Elements and compounds.
What is the difference in meaning between CoBr2 and COBr2?
CoBr2 indicates cobalt (II) bromide, while COBr2 indicates carbon monoxide bromide.
What is the definition of a molecule?
A molecule is a group of two or more atoms bonded together.
What is the classification of elements in group IA?
Group IA elements are classified as alkali metals.
Group IA Elements end with the S-subshell
What is the classification of elements in group IIA?
Group IIA elements are classified as Alkaline earth metals
Group IIA Elements end with the S-subshell
What is the classification of elements in group VIA?
Group VIA elements are classified as Chalcogens
Group VIA Elements end with the P-subshell
What is the classification of elements in group VIIA?
Group VIIA elements are classified as Halogens
Group VIIA Elements end with the P-subshell
What is the classification of elements in group VIIIA and end with which subshell?
Group VIIIA Elements are classified as Noble Gases
Group VIIIA Elements end with the S-subshell
What is the classification of elements in group B-Groups and end with which subshell?
B-Groups 4-12 are classified as transition metals.
3-12 end with the D-subshell
What periodic trends are observed for metallic character?
Metallic character increases down a group and decreases across a period
What periodic trends are observed for nonmetallic character?
nonmetallic character decreases down a group and increases across a period
What periodic trends are observed for atomic radii?
atomic radii increase down a group and decrease across a period.
Which pair of elements would exhibit the greatest similarity in their physical and chemical properties?
Elements in the same group of the periodic table.
What are the numbers of protons, neutrons, and electrons in 200 Hg?
80 protons, 120 neutrons, 80 electrons.
How do protons, neutrons, and electrons compare in charge and mass?
Protons are positively charged, neutrons are neutral, and electrons are negatively charged; protons and neutrons have similar mass, while electrons have much less mass.
Why are atoms neutral?
Atoms are neutral because they have equal numbers of protons and electrons.
If an atom contains 13 neutrons and 14 electrons, what are its mass number and number of protons?
Mass number is 27 (13 neutrons + 14 protons), and there are 14 protons.
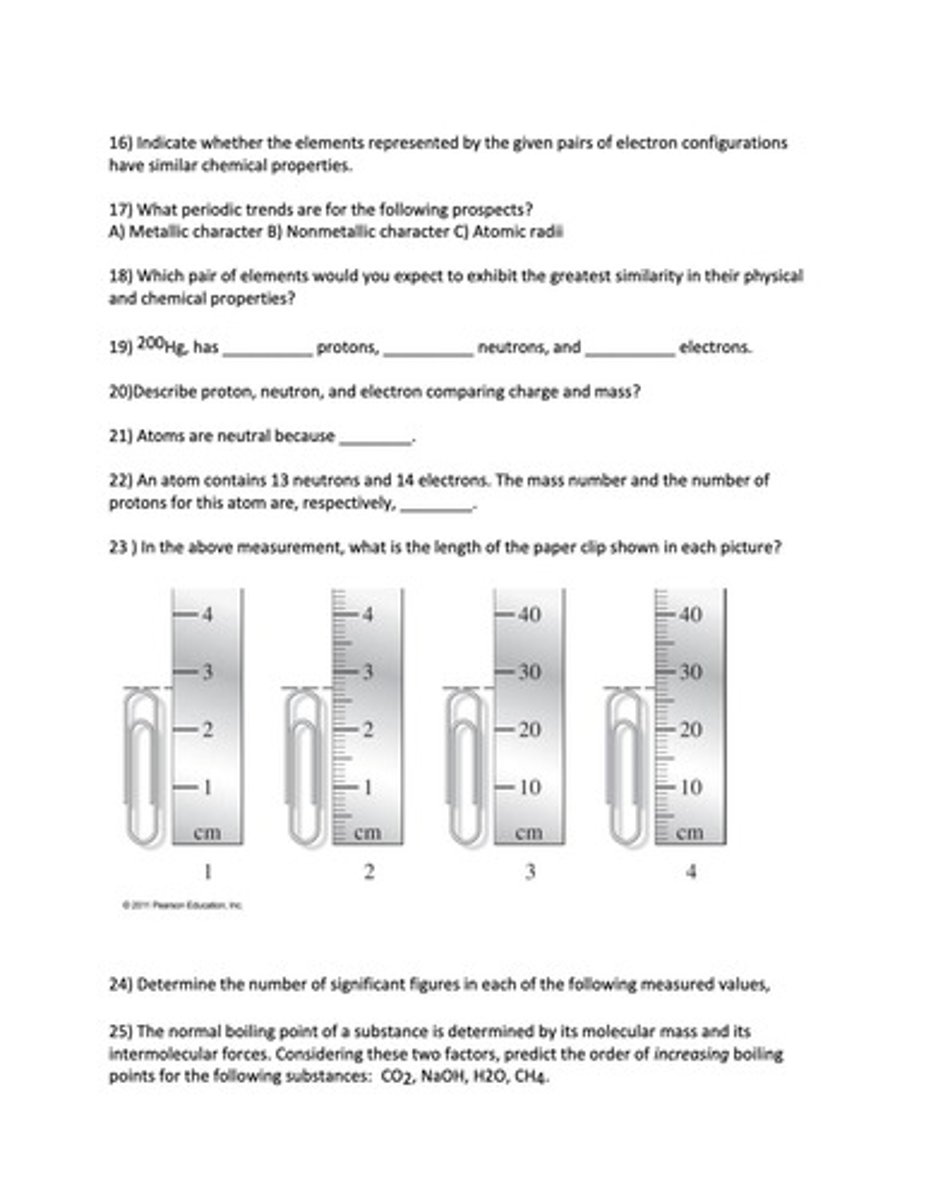
What is the length of the paper clip shown in the measurement?
1.) 2.7 cm
2.) 2.70 cm
3.) 27 cm
4.) 27.0 cm
What factors determine the normal boiling point of a substance?
Molecular mass and intermolecular forces.
What is the order of increasing boiling points for CO2, NaOH, H2O, CH4?
CH4< CO2< H2O< NaOH.
Which intermolecular force is the strongest and which is the weakest?
Hydrogen bonding is the strongest, while London dispersion forces are the weakest.
How many neutrons are in Mercury-202?
122 neutrons (202 - 80 protons).
What are isotopes?
Isotopes have the same number of protons but different numbers of neutrons.
What does the periodic law state?
When elements are arranged in order of increasing atomic number, their properties repeat at regular intervals.
How can you find the number of electrons in a complete elemental symbol?
It is the same as the atomic number or proton number.
What is the correct formula for the ionic compound containing Al3+ and NO3- ions?
Al(NO3)3.
Which pairs of elements would most likely form a covalent compound?
Pairs that are both nonmetals or close to each other on the periodic table.
How do you draw Lewis structures for simple molecules?
Refer to model kit lab reports for examples.
Which ionic compounds are named without using Roman numerals?
Ionic compounds whose metals are not in B-groups, except for fixed-charge metals like Zn and Ag.
What is the correct name for the ionic compound NaClO3?
Sodium chlorate.
What are the formulas for iron (II) sulfide and iron (III) oxide?
FeS and Fe2O3.
Which polyatomic ions do not contain oxygen?
Ammonium ion and cyanide ion.
Which polyatomic ion carries a 1+ charge?
Ammonium ion.
How many valence electrons do Group IA and IIA elements possess?
Group IA has 1 valence electron; Group IIA has 2 valence electrons.
What is the numerical value of Avogadro's number?
6.022 x 10^23.
How many Cl atoms are present in 3.16 moles of NCl3?
5.71 x 10^24 Cl atoms (3.16 moles x 3 Cl atoms/mole x 6.022 x 10^23).
Calculate the mass of 1.51 moles of Al2(Cr2O7)3.
Mass = 1060 g (1.51 moles x molar mass of Al2(Cr2O7)3.)
How many zinc atoms are in 1.98 x 10^-4 grams of zinc?
1.82 x 10^18
Which solution contains the greatest number of atoms?
(0.25 moles NaClO3) or (0.70 moles NH3)
B.) 0.70 moles NH3
(0.70) x (4) = 2.8
(0.25) x (5) = 1.25
What is the empirical formula of a compound that contains 69.94% iron and 30.06% oxygen?
Fe2O3
Balance an equation: C3H8 (g) + O2 (g) = CO2 (g) + H2O
C3H8 (g) + 5O2 (g) = 3CO2 (g) + 4H2O
Single-replacement
one element replaces another element in a compound
ex) A + BC --> AC + B
Double-replacement
a chemical reaction where two elements in different compounds trade places
ex.) AB + CD = AD + CB
Synthesis
Two reactants combine to form one product
ex.) A + B = AB
Degradation
A single substance breaks down to give two or more different substances.
ex.) AB = A + B
Combustion
A reaction in which a substance reacts with oxygen and which proceeds with the evolution of heat
reactants:
-contain oxygen
-hydrocarbons (CxHy)
Products:
-form CO2 + H2O
ex.) CH4(g) + 2O2(g) ® CO2(g) + 2H2O(g)
Given the following equation, 2 N2 + 5 O2 → 2 N2O5, 3.98 moles of nitrogen produces________ moles of N2O5.
3.98
eq. (3.98 moles N2) x (2 moles N2O5/2 moles N2) = 3.98 moles
In the following reaction, how many grams of H2O are produced if 9.88 g of O2 react?N2H4 + 3 O2 → 2NO2 + 2H2O
A. 3.71 g H2O
check the polyatomic ion table and find out the formula and charges of the following: Phosphate ion
PO₄³⁻
The concentration of a KCl solution is 0.271 molar (M, molarity). What is the density of the solution in grams per milliliter?
1.020 g/mL
check the polyatomic ion table and find out the formula and charges of the following: ammonium ion
NH₄⁺
check the polyatomic ion table and find out the formula and charges of the following: carbonate ion
CO₃²⁻
check the polyatomic ion table and find out the formula and charges of the following: nitrate ion
NO₃⁻
check the polyatomic ion table and find out the formula and charges of the following: hydroxide ion
OH⁻
Which of the following solutions contain the greatest number of particles?
a. 400.0mL of 0.10 M sodium chloride
b. 300.0 mL of 0.10 M calcium chloride
c. 200.0mL of 0.10 M iron (III) chloride
d. 200.0mL of 0.10 M potassium bromide
b. 300.0 mL of 0.10 M calcium chloride.
0.10 M x .400 L x 2 particles (NaCl) = 0.080
0.10 M x .300 L x 3 particles (CaCl2) = 0.090
0.10 M x .200 L x 4 particles (FeCl3) = 0.080
0.10 M x .200 L x 2 particles (KBr) = 0.040
check the polyatomic ion table and find out the formula and charges of the following: sulfate ion
SO₄²⁻
Calculate the pH of a solution with [OH-]=9.74 x 10^-3 M.
pH= 11.99 M
pH = 14 - pOH → pH= 14 - 2.01 = 11.99
[OH-] = 9.74 x 10^-3 M
-log(9.74 × 10^-3) = 2.01