Chemistry topic 2: chemical bonds
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
What shape is a Buckminsterfullerene molecule?(1)
Sphere
Give one use of a fullerene.(1)
drug delivery
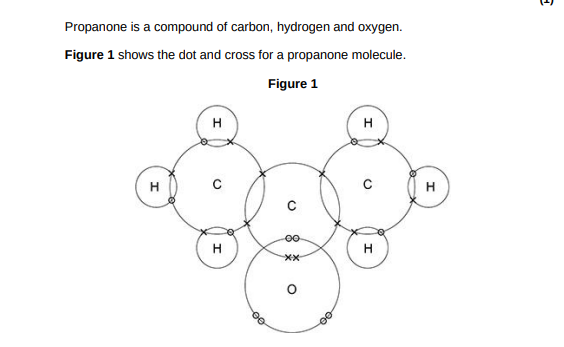
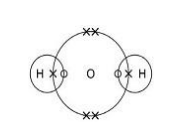
Complete Figure 2 to show a propanone molecule. Use a line to represent each single bond. Use Figure 1.(1)
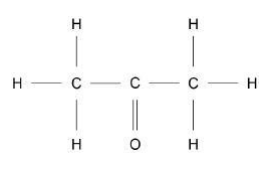
Propanone is a liquid with a low boiling point. Why does propanone have a low boiling point?(1)
The intermolecular forces are weak.
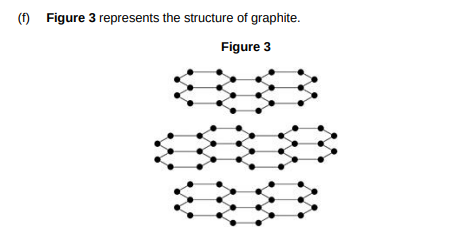
Explain why graphite is:
• a good electrical conductor
• soft and slippery.
You should answer in terms of structure and bonding.(6)
Only 3 electrons per carbon atom used in covalent bonds so one electron per carbon atom is delocalised. These delocalised electrons can move through the structure carrying electrical charge so graphite conducts electricity. Graphite is soft and slippery because there are no covalent bonds between layers so the they can slide over each other.
This question is about Group 1 elements. Give two observations you could make when a small piece of potassium is added to water.(2)
melts, floats
Complete the equation for the reaction of potassium with water. You should balance the equation. Use this: K + H2O → + (2)
2K + 2H2O → 2KOH+H2
Explain why the reactivity of elements changes going down Group 1.(4)
Reactivity increases down the group because the outer shell is further from the nucleus so there is less attraction between the nucleus and the outer shell therefore the atom loses an electron more easily.
Sodium reacts with oxygen to produce the ionic compound sodium oxide. Oxygen is a Group 6 element. Draw a dot and cross diagram to show what happens when atoms of sodium and oxygen react to produce sodium oxide.(4)
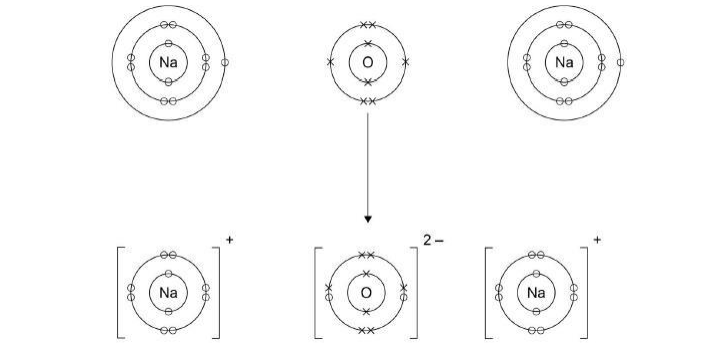
Why is oxygen described as being reduced in the reaction between sodium and oxygen?(1)
Oxygen gains electrons
Explain why sodium oxide has a high melting point.(3)
Because it is a giant structure with strong electrostatic forces of attraction between oppositely charged ions so large amounts of energy is needed to break the bonds.
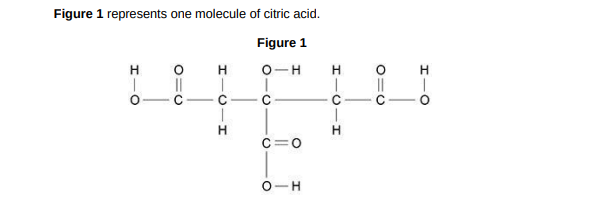
Complete the molecular formula of citric acid. Use Figure 1. C6H_O_(1)
C6H8O7
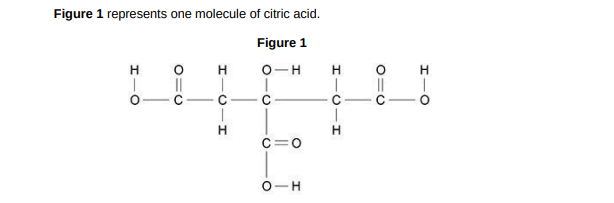
What type of bonding is shown in Figure 1?(1)
covalent
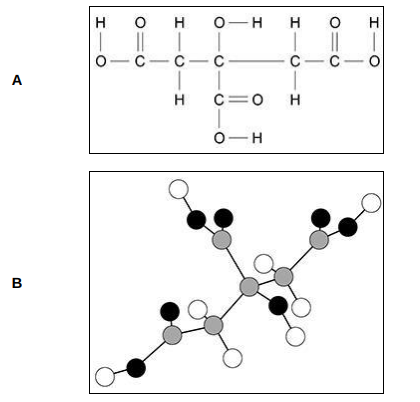
Give two advantages of representation A compared with representation B. Advantages of A:(2)
1)shows which atoms are which element
2)shows single and double bonds
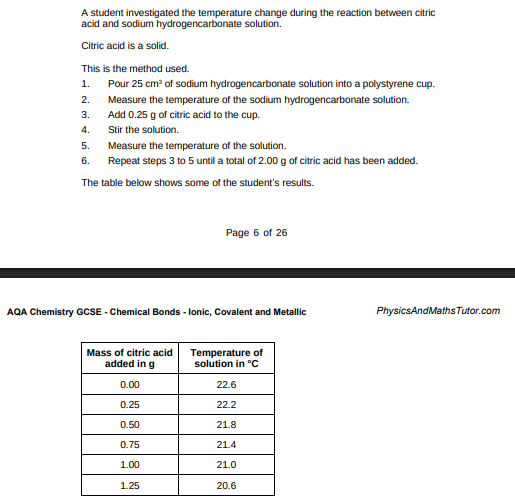
How do the results in table above show that the reaction is endothermic?(1)
temperature decreases
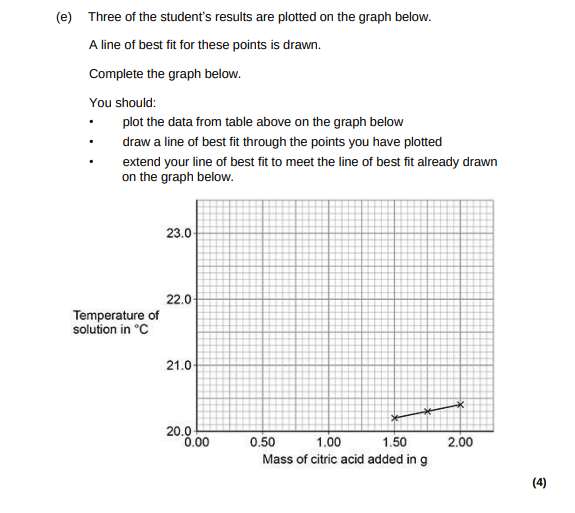
(4)
all six points plotted correctly, allow a tolerance of ± ½ small square, allow 1 mark for four / five points plotted correctly, line of best fit extrapolation to meet the printed line
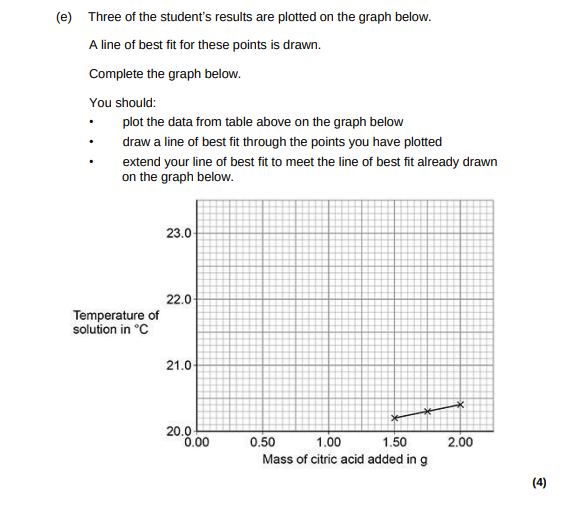
Determine the overall temperature change for the reaction. Use the graph above.(2)
22.6 – 20.2 = 2.4 (°C)

What is the dependent variable in this investigation?(1)
Temperature of solution

Which two substances have intermolecular forces between particles?(2)
Water,polyethene
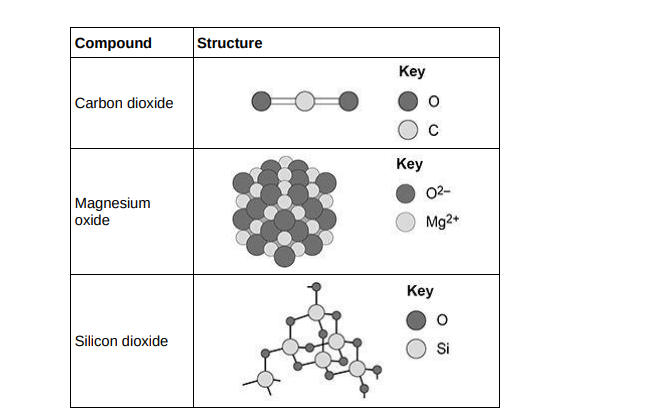
Compare the structure and bonding of the three compounds:
• carbon dioxide
• magnesium oxide
• silicon dioxide.
(6)
Carbon dioxide and silicon dioxide are made up of atoms while magnesium oxide is made of ions. Carbon dioxide and silicon dioxide are also made up of 2 non-metals meaning bonds are covalent and they share electrons while magnesium oxide is made up of a metal and non-metal meaning bonds are ionic and electrons transferred from magnesium to oxygen.
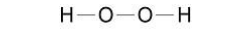
What is the correct formula of hydrogen peroxide?(1)
H2O2
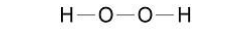
Which type of bonding is shown in Figure 1?(1)
Covalent
Hydrogen peroxide decomposes in the presence of a catalyst. Which elements are often used as catalysts?(1)
Transition metals
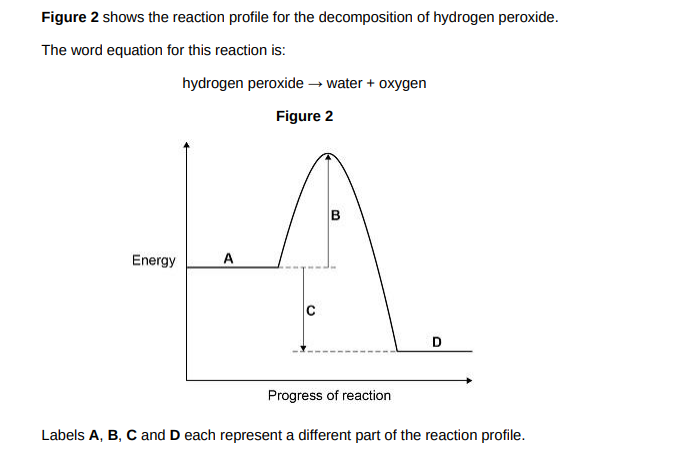
Which label shows the activation energy?(1)
B
Which label shows the energy of hydrogen peroxide?(1)
A
The decomposition of hydrogen peroxide gives out energy to the surroundings. What type of reaction is this?
Exothermic
(2)
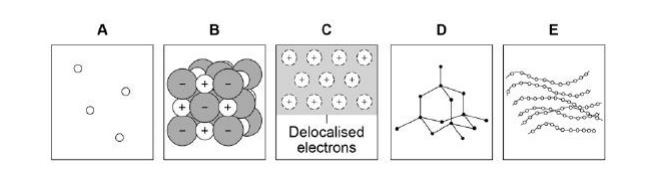
Which diagram shows a gas?(1)
A
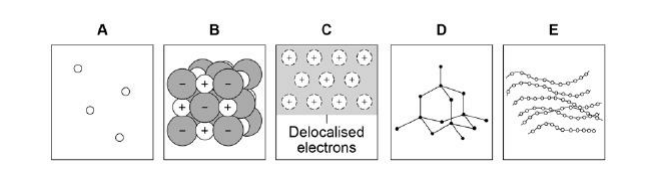
Which diagram shows the structure of diamond?(1)
D
Which diagram shows a metallic structure?(1)
C
Which diagram shows a polymer?(1)
E
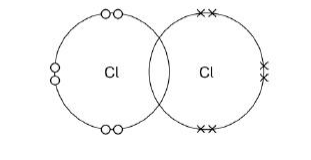
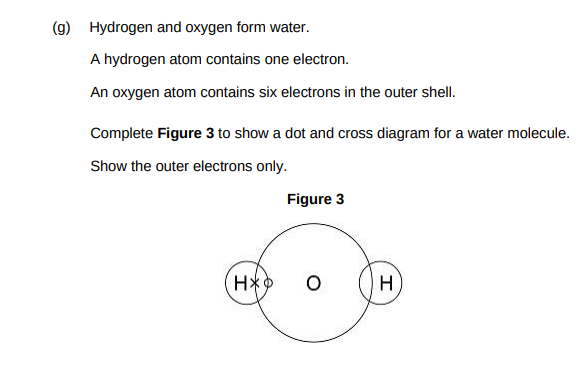
Complete the dot and cross diagram. Show only the electrons in the outer shell.(1)
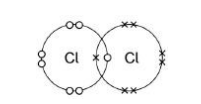

What is the reason for chlorine’s low boiling point?(1)
Weak forces between molecules
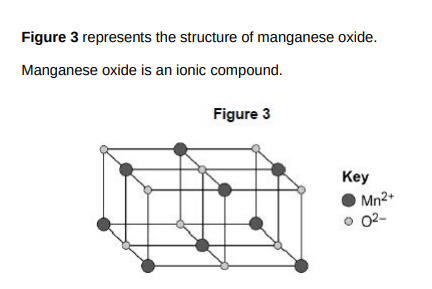
Determine the empirical formula of manganese oxide(1)
MnO

Why does manganese oxide conduct electricity as a liquid?(1)
Ions move around in the liquid
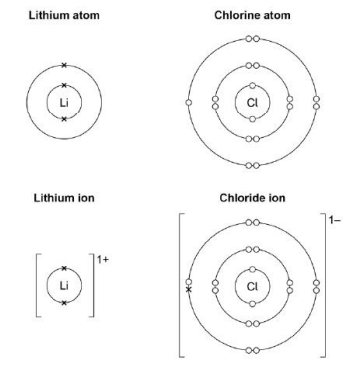
Describe what happens when a lithium atom reacts with a chlorine atom. Answer in terms of electrons(4)
Lithium loses one electron and cholorine gains one electron to form positive and negative ions.

(3)
161/81+98 ×100 = 89.944134 = 89.9%
Method 1 gives a higher percentage atom economy for making zinc sulfate than Method 2. Give a reason why it is important to use a reaction with a high atom economy.(1)
Less waste
A student uses 50 cm3 of a zinc sulfate solution of 80 g/dm3 What mass of zinc sulfate is dissolved in 50 cm3 of this zinc sulfate solution?(2)
50cm3 = 0.05dm3, 0.05×80 = 4g
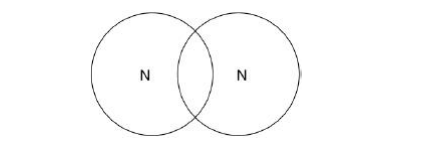
Complete the dot and cross diagram to show the covalent bonding in a nitrogen molecule, N2. Show only the electrons in the outer shell.(2)
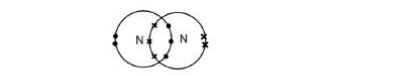
Explain why nitrogen is a gas at room temperature. Answer in terms of nitrogen’s structure(3)
It has weak forces between molecules which need little energy to overcome
Graphite and fullerenes are forms of carbon. Graphite is soft and is a good conductor of electricity. Explain why graphite has these properties. Answer in terms of structure and bonding(4)
Each carbon atom forms three covalent bonds forming layers that slide over each oterh so graphite is soft and it conducts electricity because of delocalised electrons.
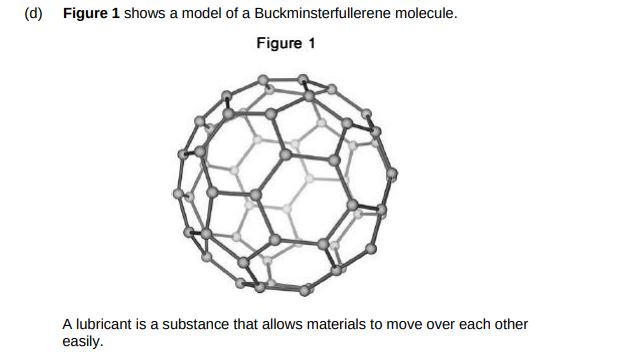
Suggest why Buckminsterfullerene is a good lubricant.(2)
Molecules are sperical so they can roll
A silver nanocrystal is a cube of side 20 nm Calculate the surface area to volume ratio of the nanocrystal.
Surface area: 20×20×6 = 2400, volume: 20cubed = 8000, Ratio: 1:3.33
Silver nanoparticles are sometimes used in socks to prevent foot odour. Suggest why it is cheaper to use nanoparticles of silver rather than coarse particles of silver.(2)
Nanoparticles have a larger surface area to volume ratio so less can be used for the same effect.
Describe what happens when two atoms of potassium react with one atom of sulfur. Give your answer in terms of electron transfer. Give the formulae of the ions formed(5)
2 pottasium atoms lose 1 electron each to form 1+ ions while sulfur gains 2 electrons to form 2- ions so the electrons are transferred from pottasium to sulfur.
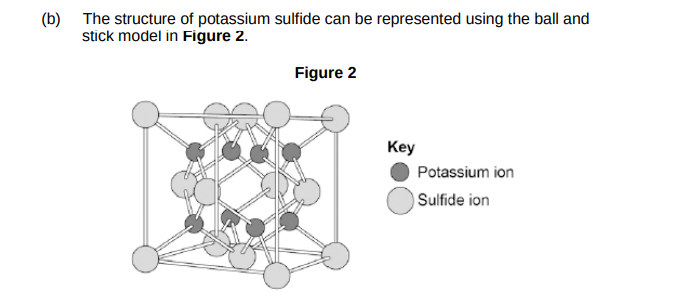
The ball and stick model is not a true representation of the structure of potassium sulfide. Give one reason why.(1)
There are no gaps between between potassium ions and sulfide ions
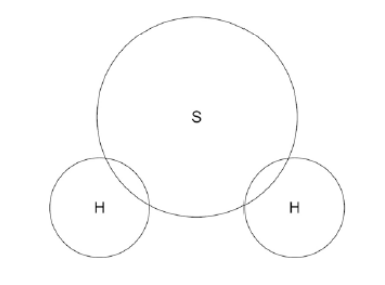
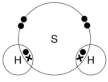
Complete the dot and cross diagram to show the covalent bonding in a molecule of hydrogen sulfide. Show the outer shell electrons only(2)
Calculate the relative formula mass (Mr) of aluminium sulfate Al2(SO4)3 Relative atomic masses (Ar): oxygen = 16; aluminium = 27; sulfur = 32(2)
342
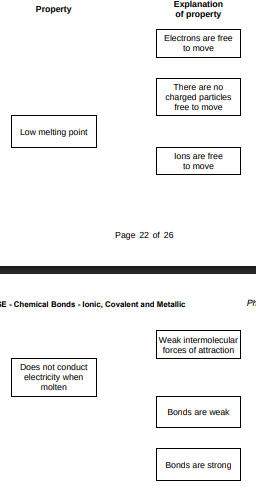
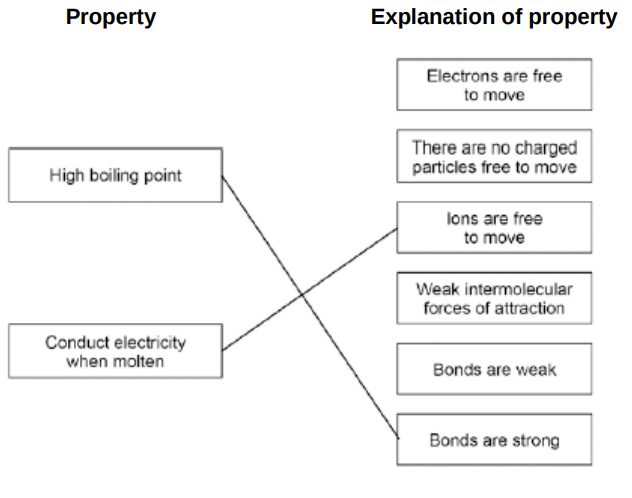
Draw one line from each property to the explanation of the property.(2)
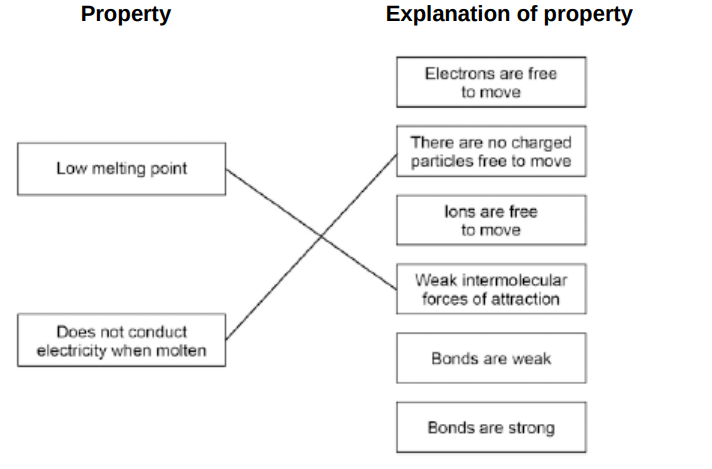
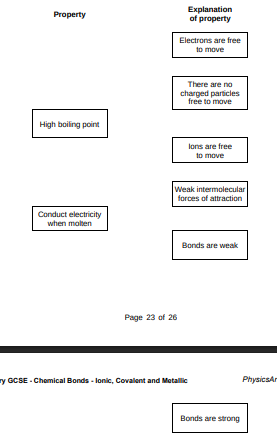
Draw one line from each property to the explanation of the property.(2)
In which group of the periodic table is fluorine?(1)
Group 7
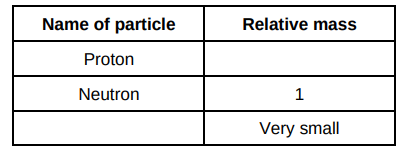
Complete the table below to show the particles in an atom and their relative masses.(2)
1,Electron

Atoms of fluorine with different numbers of neutrons are called ________________(1)
isotopes
Sodium reacts with fluorine to produce sodium fluoride. Complete the word equation for this reaction. sodium + ___________________ → _________________(1)
Fluorine, sodium fluoride
Substances in which atoms of two or more different elements are chemically combined are called _____________________ .(1)
compounds

The relative formula mass (Mr), in grams, of sodium fluoride is one _______________ of the substance.(1)
mole
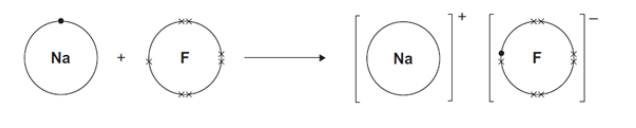
Describe, as fully as you can, what happens when sodium reacts with fluorine to produce sodium fluoride.(4)
sodium atom loses 1 electron to from Na+ ion while fluorine atom gains 1 one electron to form F- ion.
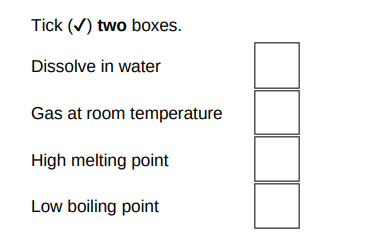
Sodium fluoride is an ionic substance. What are two properties of ionic substances?(2)
High melting point, dissolve in water