Topic 9 - Separate Chemistry 2 ✅
1/38
Earn XP
Description and Tags
imported from quizlet. whoever you are THANK YOU
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
QUALITATIVE ANALYSIS: TESTS FOR IONS
9.1C Explain why the test for any ion must be unique
You would never be able to know which specific ion it was if more than one ion gave the same result
9.2C Describe flame tests to identify the following ions in solids:
a) lithium ion, Li+
b) sodium ion, Na+
c) potassium ion, K+
d) calcium ion, Ca+
e) copper ion, Cu2+
Method for flame test
To test a substance, dip the clean flame test loop in a solution of the ions and hold at the edge of a blue flame.
Clean the flame test loop in acid each time, rinse with water and check it is clean in the Bunsen burner flame (so remnants of other ions dont interfere with the results)
Results (flame colour):
lithium- red
sodium- yellow
potassium- lilac
calcium- orange-red
copper- blue-green
9.3C Describe tests to identify the following ions in solids or solutions as appropriate:
a) aluminium ion, Al3+
b) calcium ion, Ca2+
c) copper ion, Cu2+
d) iron(II) ion, Fe2+
e) iron(III) ion, Fe3+
f) ammonium ion, NH4+
using sodium hydroxide solution
method -
The sample solution is placed in a test tube and a few drops of dilute sodium hydroxide solution are added. Some metal ions form coloured hydroxide precipitates.
Results (colour of precipitate) -
aluminium ions- white
calcium ions- white
copper ions- blue
iron(II) ions- green
iron(III) ions- brown
ammonium ions- no change
After adding excess sodium hydroxide
aluminium- the precipitate re-dissolves
calcium- no change
9.4C Describe the chemical test for ammonia
Add sodium hydroxide and heat, producing ammonia gas (NH3)
NH4+(aq) + OH-(aq) -> H2O(l) + NH3(g)
Hold damp red litmus paper above the sample. Ammonia gas will turn damp red litmus paper blue.
Ammonia has a characteristic sharp smell that can be used to identify it
9.5C Describe tests to identify the following ions in solids or solutions as appropriate:
a) carbonate ion, CO32-
b) sulfate ion, SO42-
c) chloride ion Cl-, bromide ion Br-, iodide ion I-
carbonate ion:
add a few drops of dilute acid
effervescence will occur as carbon dioxide is given off
bubble the gas through limewater, if it turns cloudy, the gas is carbon dioxide and carbonate ions are present
sulfate ion:
add dilute hydrochloric acid (HCl)
add barium chloride solution (BaCl2)
if sulfate ions are present, a white precipitate forms
halide ions:
add dilute nitric acid (HNO3)
add silver nitrate solution (AgNO3)
coloured precipitates formed if halide ions are present:
chloride - white
bromide - cream
iodide - yellow
9.6C Core Practical: Identify the ions in unknown salts, using the tests for the specified cations and anions in 9.2C, 9.3C, 9.4C, 9.5C
9.7C Identify the ions in unknown salts, using results of the tests above
9.8C Describe that instrumental methods of analysis are available and that these may improve sensitivity, accuracy and speed of tests
Compared with simple chemical tests, instrumental methods (e.g. flame photometer) offer improved:
sensitivity- they can detect and analyse very small amounts of different substances
accuracy- they measure amounts of different substances very accurately
speed of tests- they carry out each analysis quickly, and the machines can run all the time
9.9C Evaluate data from a flame photometer:
a) to determine the concentration of ions in dilute solution using a calibration curve
b) to identify metal ions by comparing the data with reference data
(no knowledge of the instrument or how it works is required)
a)
b) identify the metal ions present in the sample by comparing the spectrum with a reference spectrum from a known substance. If the lines in a spectrum match the lines in a reference spectrum, the ions must be the same
HYDROCARBONS
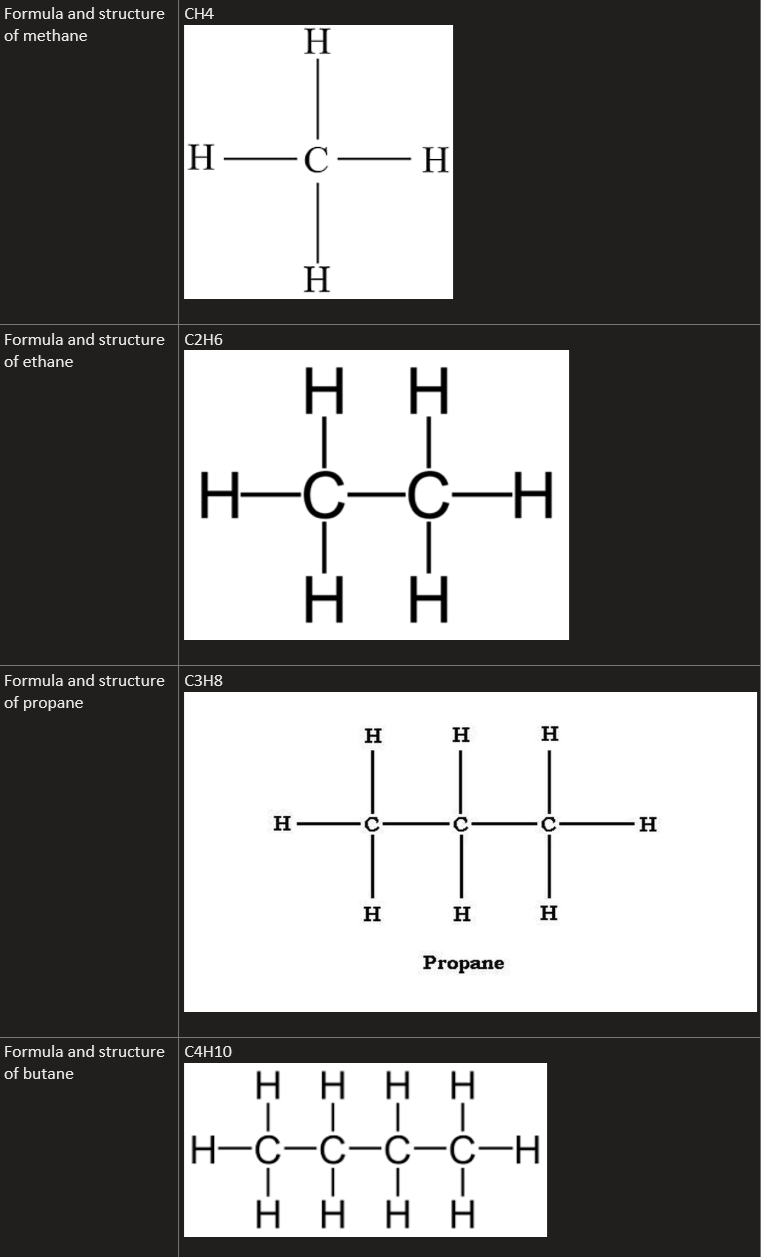
9.10C Recall the formulae of molecules of the alkanes, methane, ethane, propane and butane and draw the structures of these molecules, showing all covalent bonds
methane: CH4
ethane: C2H6
propane: C3H6
butane: C4H10
general formula is: CnH2n+2
9.11C Explain why the alkanes are saturated hydrocarbons
They contain only hydrogen and carbon atoms and the carbon atoms are joined together by single bonds.
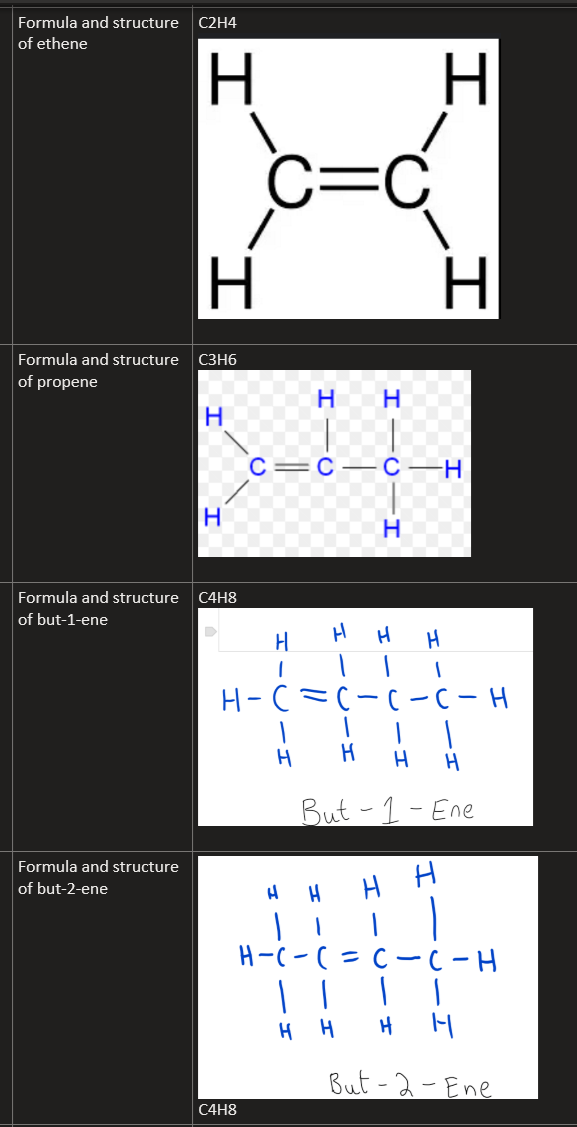
9.12C Recall the formulae of molecules of the alkenes, ethene, propene, butene, and draw the structures of these molecules, showing all covalent bonds (but-1-ene and but-2-ene only)
ethene: C2H4
propene: C3H6
butene: C4H8
but-1-ene has the carbon double bond in the first/last spot
but-2-ene has the carbon double bond in the middle spot
general formula: CnH2n
9.13C Explain why the alkenes are unsaturated hydrocarbons
They contain only hydrogen and carbon atoms and also a double bond between carbon atoms (C=C)
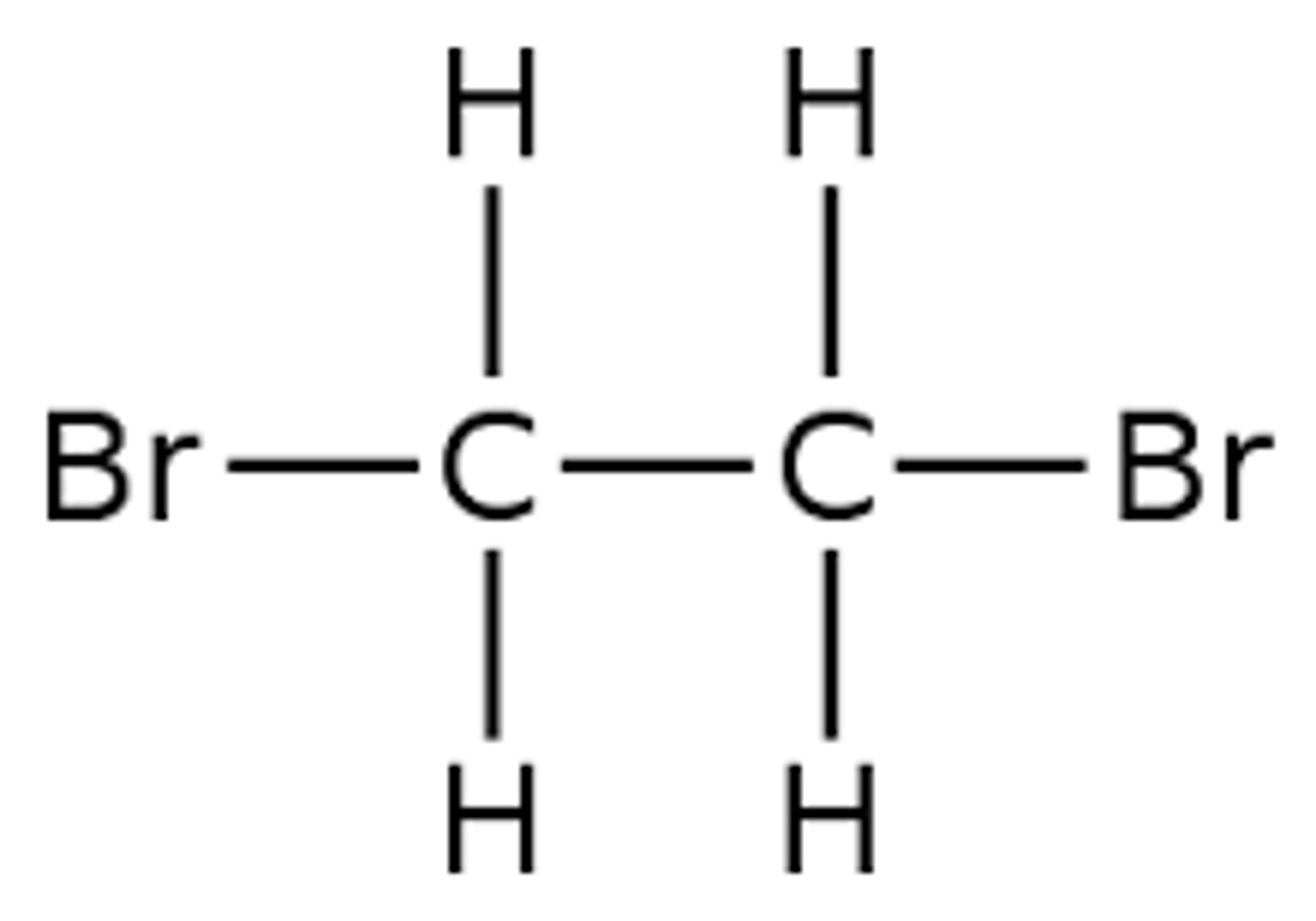
9.14C Recall the addition reaction of ethene with bromine, showing the structures of reactants and products, and extend this to other alkenes
ethene + bromine -> dibromoethane
C2H4 + Br2 -> C2H4Br2
9.15C Explain how bromine water is used to distinguish between alkanes and alkenes
Due to double carbon bond, alkenes react with bromine water and decolourise it.
Orange- shake- colourless.
alkanes dont have double carbon bonds so the bromine water remains orange
9.16C Describe how the complete combustion of alkanes and alkenes involves the oxidation of the hydrocarbons to produce carbon dioxide and water
complete combustion of alkenes/alkanes:
carbon is oxidised to carbon dioxide
hydrogen is oxidised to water vapour
e.g.
ethene + oxygen -> carbon dioxide + water
C2H4 + 3O2 -> 2CO2 + 2H2O
POLYMERS
9.17C What is a polymer?
A substance of high average relative molecular mass
made up of small repeating units called monomers
9.18C Describe:
a) how ethene molecules can combine together in a polymerisation reaction
b) the name of the addition polymer
a)
The carbon-carbon double bond (C=C) breaks open, allowing the carbon atoms to form single bonds with other molecules.
So, each ethene molecule can link with other ethene molecules.
This forms a long chain of repeating units.
b)
the addition polymer formed is called poly(ethene)
9.19C Describe how other addition polymers can be made and some examples of them
addition polymers are made by combining monomer molecules that contain C=C bonds
the double bond breaks open and form single bonds with other monomers
propene → poly(propene)
chloroethene → poly(chloroethene)
tetrafluoroethene → poly(tetrafluoroethene)
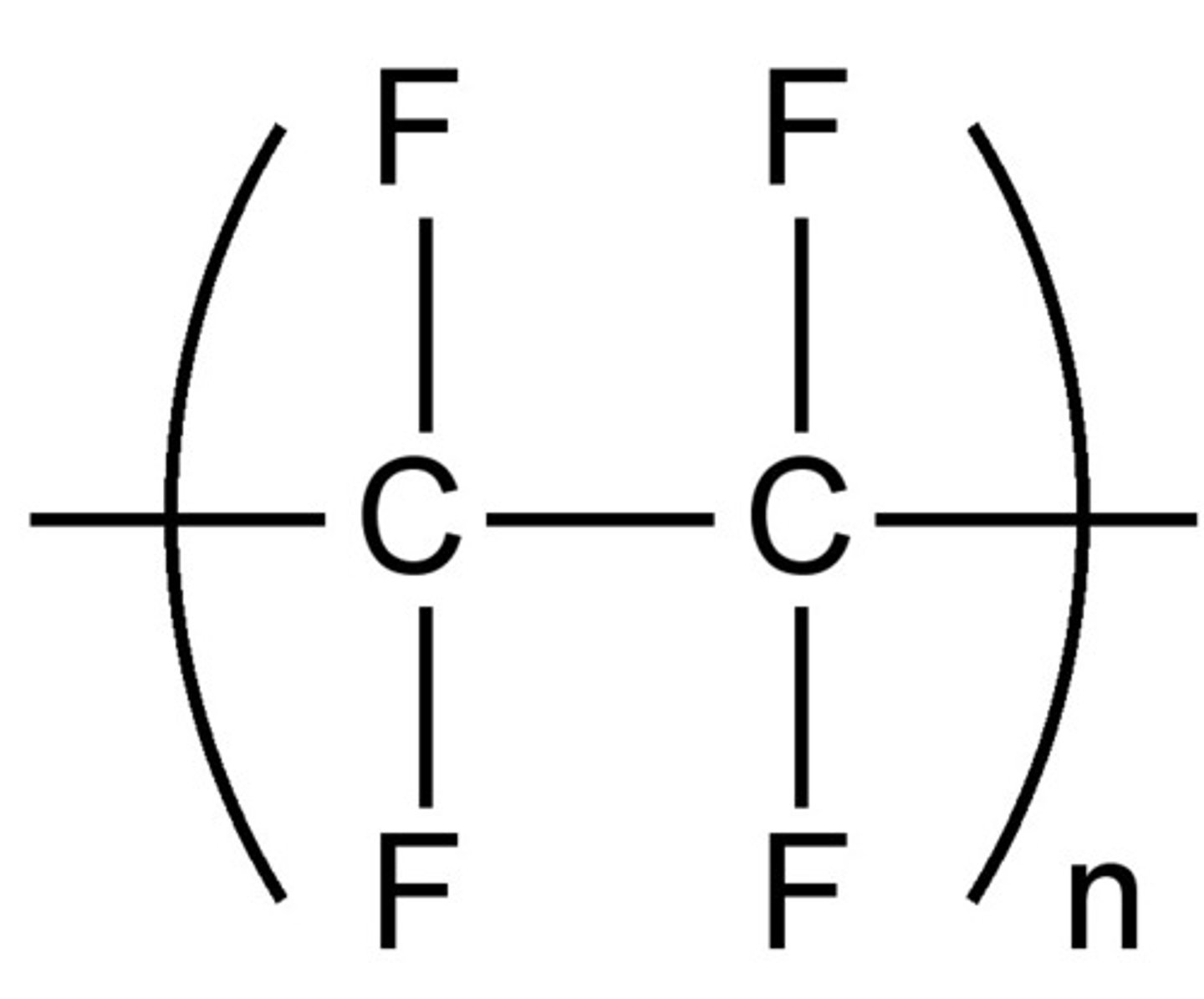
9.20C Deduce the structure of a monomer from the structure of an addition polymer and vice versa
Look for the repeating unit – it's usually drawn in brackets with a subscript n. e.g. [–CH₂–CH₂–]ₙ
To find the monomer, add a double bond back between the two carbon atoms in the repeat unit.
Always check the side groups (like –Cl, –CH₃, etc.) to make sure you correctly identify the monomer.
9.21C Explain how the uses of polymers are related to their properties for: poly(ethene),
poly(propene),
poly(chloroethene) (PVC)
poly(stryene)
poly(tetrafluoroethene) (PTFE)
poly(ethene)
properties: does not dissolve in water, light, flexible
uses: plastic bags, washing up bowls
poly(propene)
properties: flexible, durable, strong
uses: milk bottle crates
poly(styrene)
properties: light, poor conductor of heat
use: packaging
poly(chloroethene) / pvc
properties: durable, electric insulator, waterproof, easily moulded
uses: pipes, guttering, insulation of electric cables
poly(tetrafluoroethene) / teflon / ptfe
properties: thermal insulator, provides a smooth surface, high MP
uses: non stick coating for frying pans
9.22C Explain:
a) why polyesters are condensation polymers
b) how a polyester is formed when a monomer molecule containing two carboxylic acid groups is reacted with a monomer molecule containing two alcohol groups
c) what else is formed every time an ester is formed
a) because they are made from a condensation reaction, not addition. Two different types of monomers are joined together, producing water each time.
b) To make a polyester, we need:
A monomer with two carboxylic acid groups (–COOH)
A monomer with two alcohol groups (–OH)
When these react:
The –COOH group from one monomer reacts with the –OH group from the other.
An ester link (–COO–) is formed.
c) A molecule of water (H₂O) is produced in the process.
one repeating unit produces two water molecules so would be 2nH2O
general equation: dicarboxylic acid monomer + diol monomer -> condensation polymer + water
9.23C Describe some problems associated with polymers including the:
a) availability of starting materials
b) persistence in landfill sites, due to non-biodegradability
c) gases produced during disposal by combustion
d) requirement to sort polymers so that they can be melted and reformed into a new product
a) most polymers are made from alkenes, which come from crude oil - a finite, non-renewable resource
b) many polymers (esp addition polymers) are non-biodegradable so end up in landfills for hundreds of years since it takes a long time to break down. We are running out of landfill sites. (the polymers last a long time tho)
c) when incinerated, polymers may produce harmful gases:
carbon dioxide (greenhouse gas)
toxic gases (e.g. hydrogen chloride from burning PVC)
these can be harmful to the environment and human health
also a waste of finite resources
d) they are hard to recycle because:
different polymers melt at different temperatures and have different properties.
so you must sort them by type before recycling
This is time-consuming, costly, and not always done properly. Some polymers cannot be recycled
two ways to recycle:
melting and reforming into new objects
break down into raw materials
9.24C Evaluate the advantages and disadvantages of recycling polymers
advantages:
conserves (finite) raw materials
reduces waste in landfills
disadvantages:
collecting and sorting is expensive and time-consuming
quality of recycled plastics degrades
9.25 in terms of polymers:
a) what is DNA
b) what is starch
c) what are proteins
a) DNA is a polymer made from four different monomers called nucleotides
b) starch is a polymer based on sugars, made from many sugar monomers joined together
c) proteins are polymers based on amino acids
ALCOHOLS AND CARBOXYLIC ACIDS
9.26C Recall the formulae of molecules of the alcohols, methanol, ethanol, propanol (propan-1-ol only) and butanol (butan-1-ol only), and draw the structures of these molecules, showing all covalent bonds
general formula: CₙH₂ₙ₊₁OH
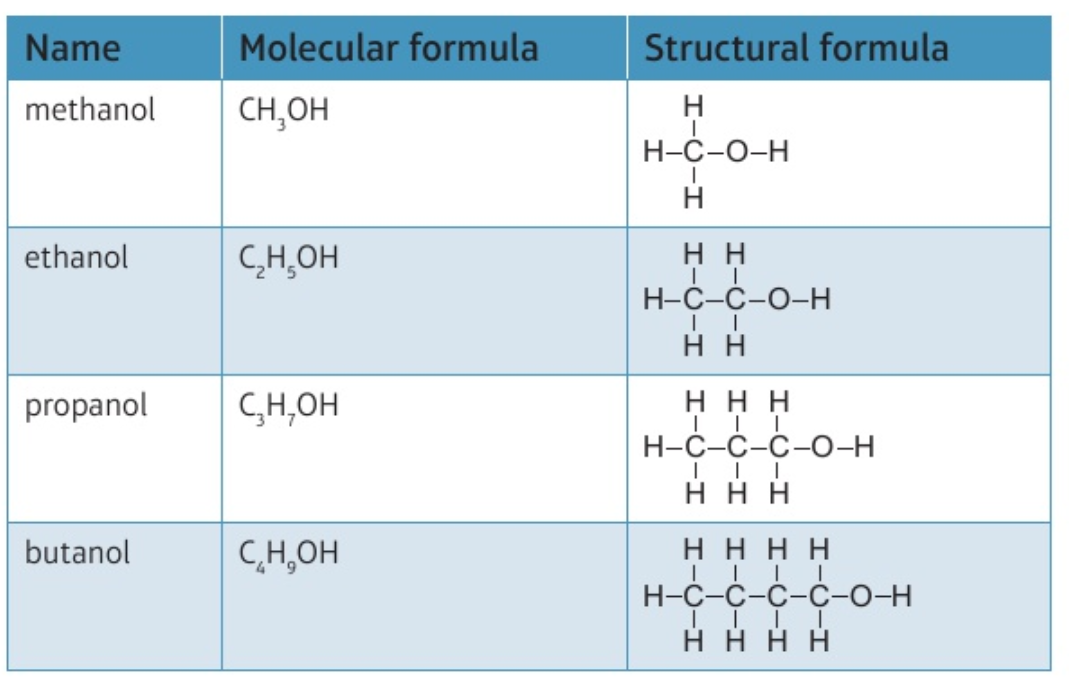
9.27C what is the functional group in alcohols?
what are alkenes dehydrated to form?
functional group: -OH (hydroxyl)
alcohols can be dehydrated to form alkenes
Alcohol → Alkene + Water
E.g. Ethanol → Ethene + H₂O
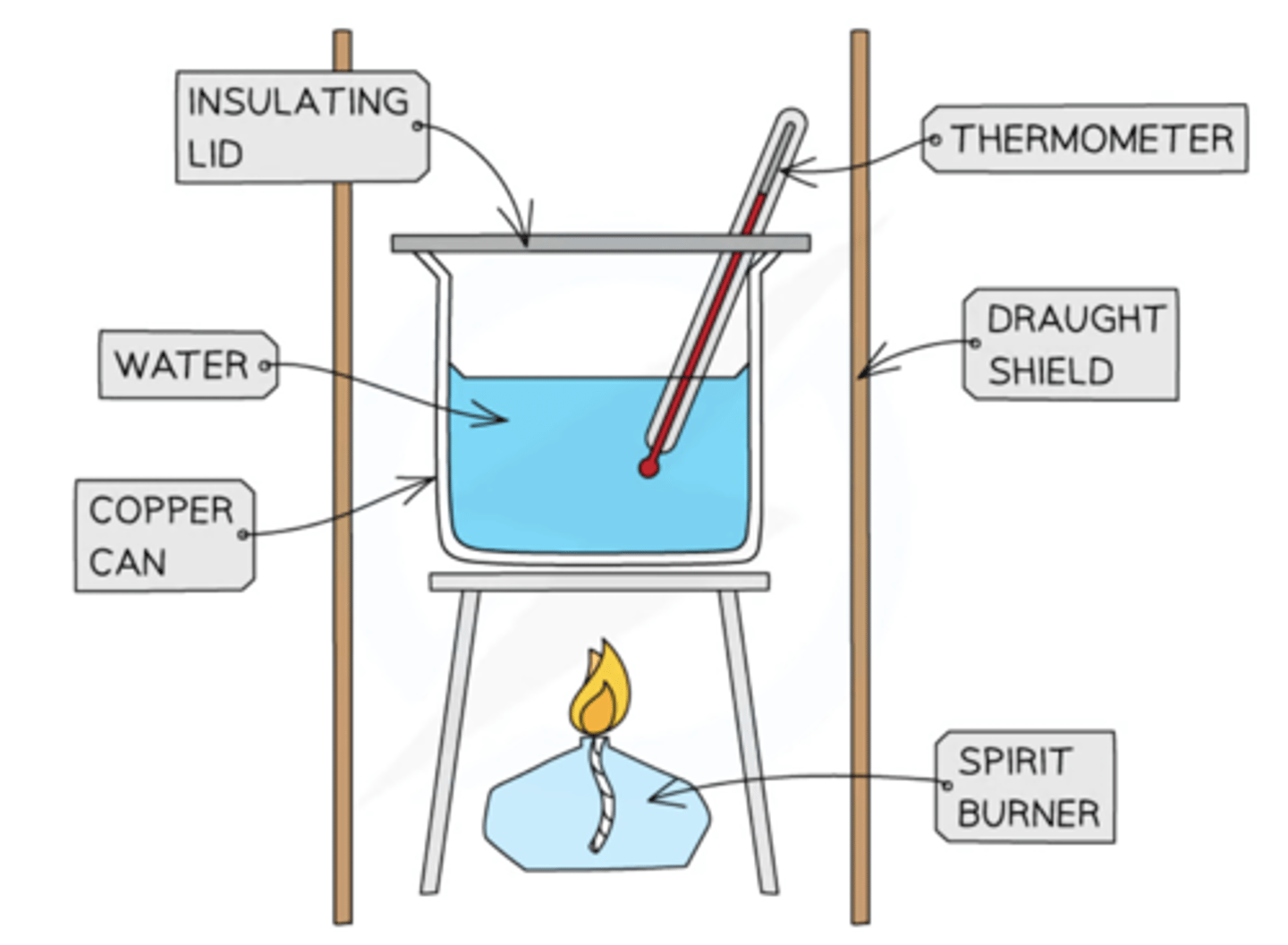
9.28C Core Practical: Investigate the temperature rise produced in a known mass of water by the combustion of the alcohols ethanol, propanol, butanol and pentanol
method, safety, variables, error
1) Measure the mass of the alcohol burner and cap. Record the mass and name of alcohol
2) Place the alcohol burner at the centre of a heat resistant mat
3) Use a measuring cylinder to add 100cm3 of cold water to a conical flask
4) Measure and record the initial temp of the water and clamp the flask above the alcohol burner
5) Light the wick of the burner and allow the water to heat up by about 40°C
6) Replace the cap on the burner and measure and record the final temp of the water
7) Measure and record the mass of the alcohol burner and cap
8) Calculate the mass of the alcohol burned (the change in mass) to produce a 1°C rise in temp
9) Repeat the experiment using different alcohols and cold fresh water
safety:
wear goggles
use a lit split to light the spirit burner instead of a match
dont move the spirit burner when lit
ensure room is well ventilated
Possible sources of error in this experiment are:
heat loss to surroundings,
not all flames are the same height from different spirit burners,
incomplete combustion and evaporation of alcohol while weighing.
control variables:
mass/volume of water,
height of copper can above wick,
height of flame,
shape of copper container
the number of moles of alcohol.
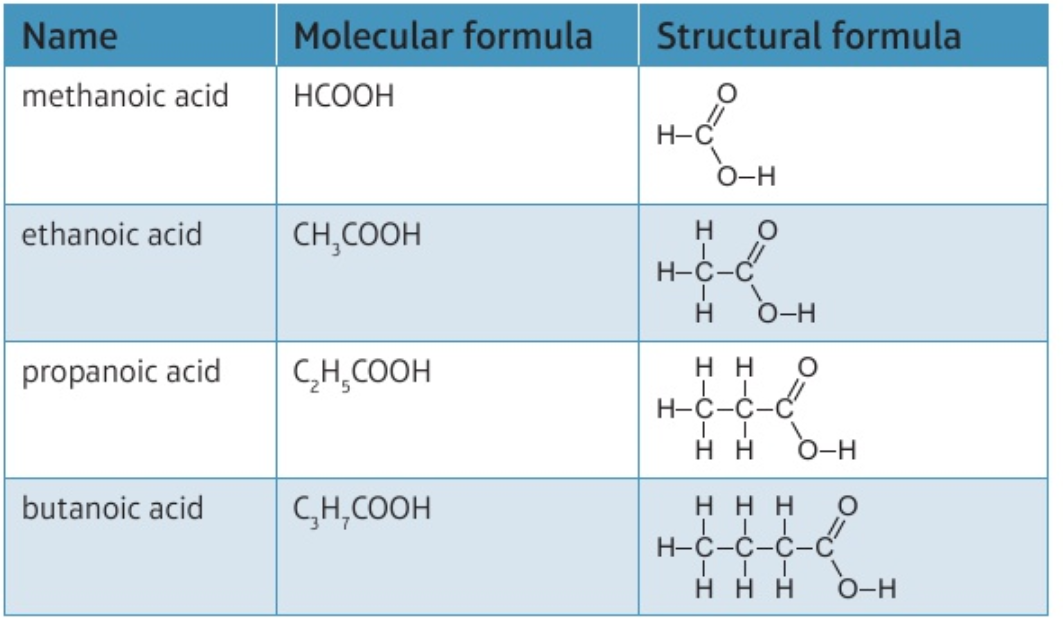
9.29C Recall the formulae of molecules of the carboxylic acids, methanoic, ethanoic, propanoic and butanoic acids, and draw the structures of these molecules, showing all covalent bonds
methanoic: HCOOH
ethanoic: CH₃COOH
propanoic: C₂H₅COOH
butanoic: C₃H₇COOH
9.30C what is the functional group of carboxylic acids?
what are the properties of carboxylic acids?
functional group: -COOH
Carboxylic acids have the typical chemical properties of acids.
They can:
react with carbonates to produce a salt, water and carbon dioxide
react with magnesium and other reactive metals to produce a salt and hydrogen
dissolve in water to produce acidic solutions
They are weak acids since they partially dissociate into hydrogen ions when they dissolve in water.
9.31C What can ethanol be oxidised to produce?
Ethanol can be oxidised to produce ethanoic acid
ethanol + oxidising agent -> ethanoic acid + water
other alcohols are the same.
9.32C what are the properties of members of a homologous series
carboxylic acids (in a homologous series):
have the same functional group, -COOH so have similar reactions
have similar chemical properties
differ in the molecular formulae of neighbouring members by CH2
show a gradual variation in physical properties, such as boiling points
9.33C Describe the production of ethanol by fermentation of carbohydrates in aqueous solution, using yeast to provide enzymes
Fermentation is the biological process
where yeast converts carbohydrates (usually glucose) into ethanol (alcohol) and carbon dioxide (CO₂)
It happens in the absence of oxygen (anaerobic conditions)
yeast is a single-celled fungus. It provides enzymes for fermentation to happen (the enzymes breaks down sugars):
C₆H₁₂O₆(aq) -> 2C₂H₅OH(aq) + 2CO₂(g)
glucose -> ethanol + carbon dioxide
The fermentation mixture must be kept warm and under anaerobic conditions
the reaction is too slow at low temps and yeast enzymes dont work at high temps.
Fermentation is a type of anaerobic respiration so if oxygen is present, aerobic respiration will occur instead → producing carbon dioxide and water only.
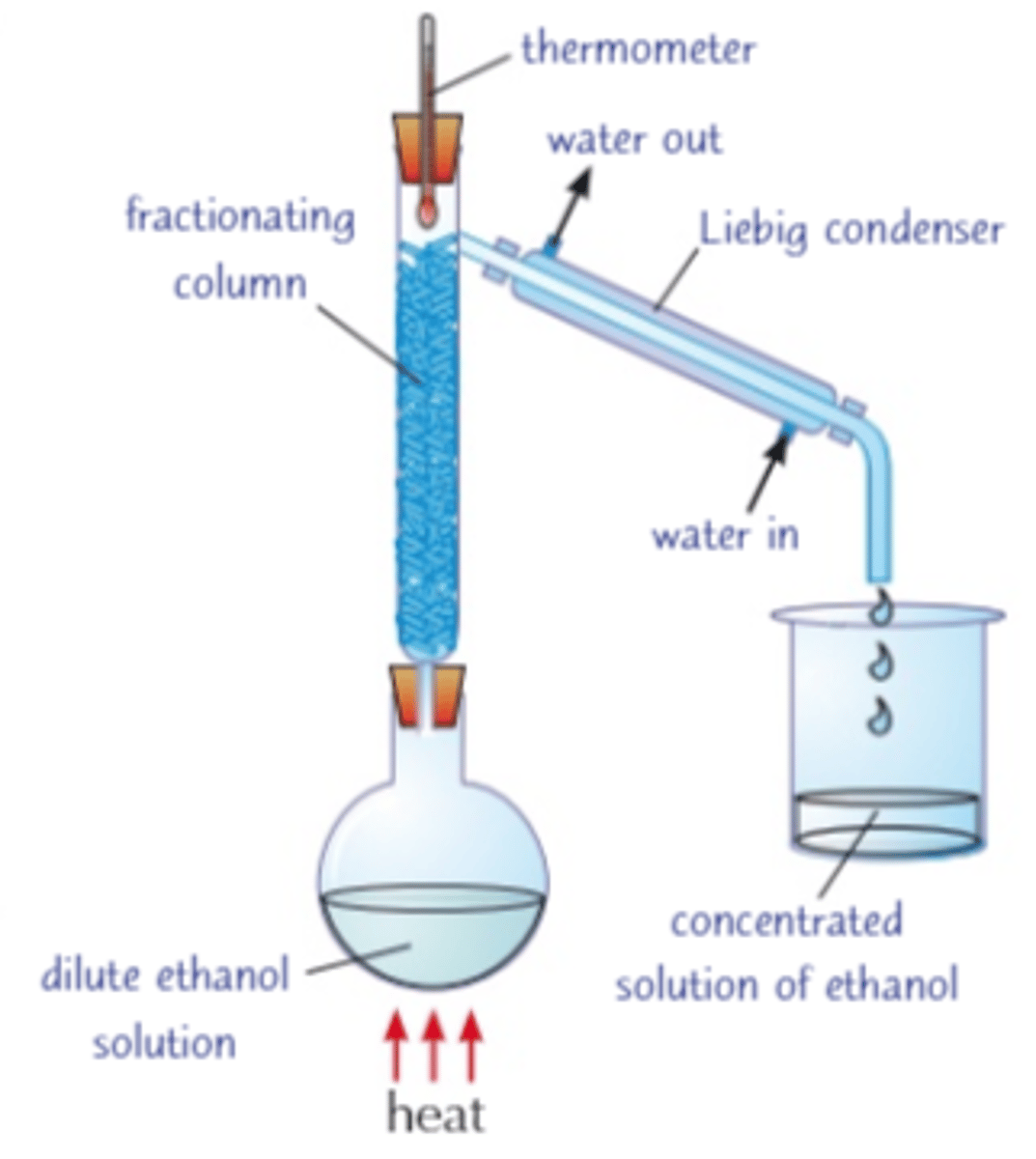
9.34C Explain how to obtain a concentrated solution of ethanol by fractional distillation of the fermentation mixture
Fractional distillation works because ethanol has a lower boiling point than water so evaporates first
and can be condensed and collected as a concentrated solution of ethanol.
it’s not possible to obtain pure ethanol by this method alone; the rest must be absorbed chemically
BULK AND SURFACE PROPERTIES OF MATTER INCLUDING NANOPARTICLES
9.35C Compare the size of nanoparticles with the sizes of atoms and molecules
smallest -> largest
proton, atom, molecule, nanoparticle
9.36C Describe how the properties of nanoparticulate materials are related to their uses
nanoparticles have a very small size (1-100nm) so a very large surface area:volume ratio.
uses of nanoparticles
catalysts
more frequent successful collisions because there is more area exposed for collisions
sunscreen
Nanoparticles of zinc oxide are used in suncream
This is because zinc oxide helps absorb UV radiation
The nanoparticle form is used so that it's easier to rub onto your skin
socks
Silver nanoparticles
more effective at killing bacteria because of their greater surface area to volume ratio.
This means there are more silver atoms in contact with the bacteria for the same amount of silver
9.37C Explain the possible risks associated with some nanoparticulate materials
nanoparticles have only been discovered recently so there could be long-term risks.
If they end up in sewage systems after being washed off in showers, nanoparticles of titanium dioxide are able to eliminate microbes which play vital roles in ecosystems
Secondly nanoparticles are small enough to penetrate cell membrane, damaging cells
9.38C Compare the physical properties of glass and clay ceramics, polymers, composites, and metals
Glass ceramics:
Properties
Hard
hard but brittle
poor conductors of heat and electricity
uses
· windows
· bottles
Clay ceramics:
properties
opaque
hard but brittle
poor conductors of heat and electricity
uses
bricks
porcelain
Polymers:
Properties
can be made transparent/translucent/opaque
poor conductors of heat and electricity
often tough and ductile
uses
plastic bags
bottles
Metals:
Properties
shiny
tough
good conductors of heat and electricity
uses
cars
bridges
electrical cables
9.39C Explain why the properties of a material make it suitable for a given use and use data to select materials appropriate for specific uses
the uses of glass ceramics, clay ceramics, polymers, metals
glass ceramics:
window glass
bottles
clay ceramics:
bricks
china
porcelain
polymers:
bottles
crates
carrier bags
metals:
cars
bridges
electrical cables