NON COVALENT INTERACTIONS
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
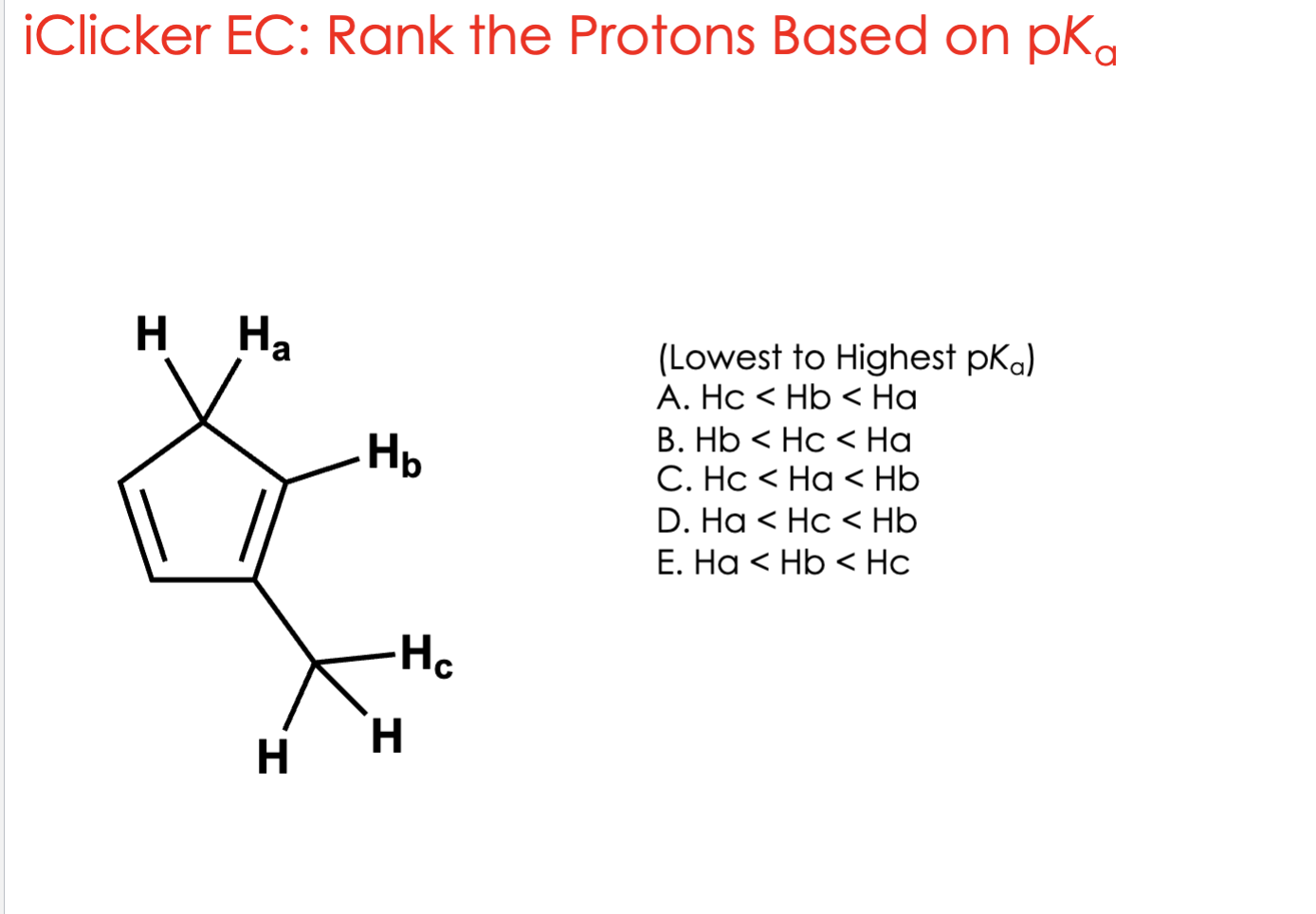
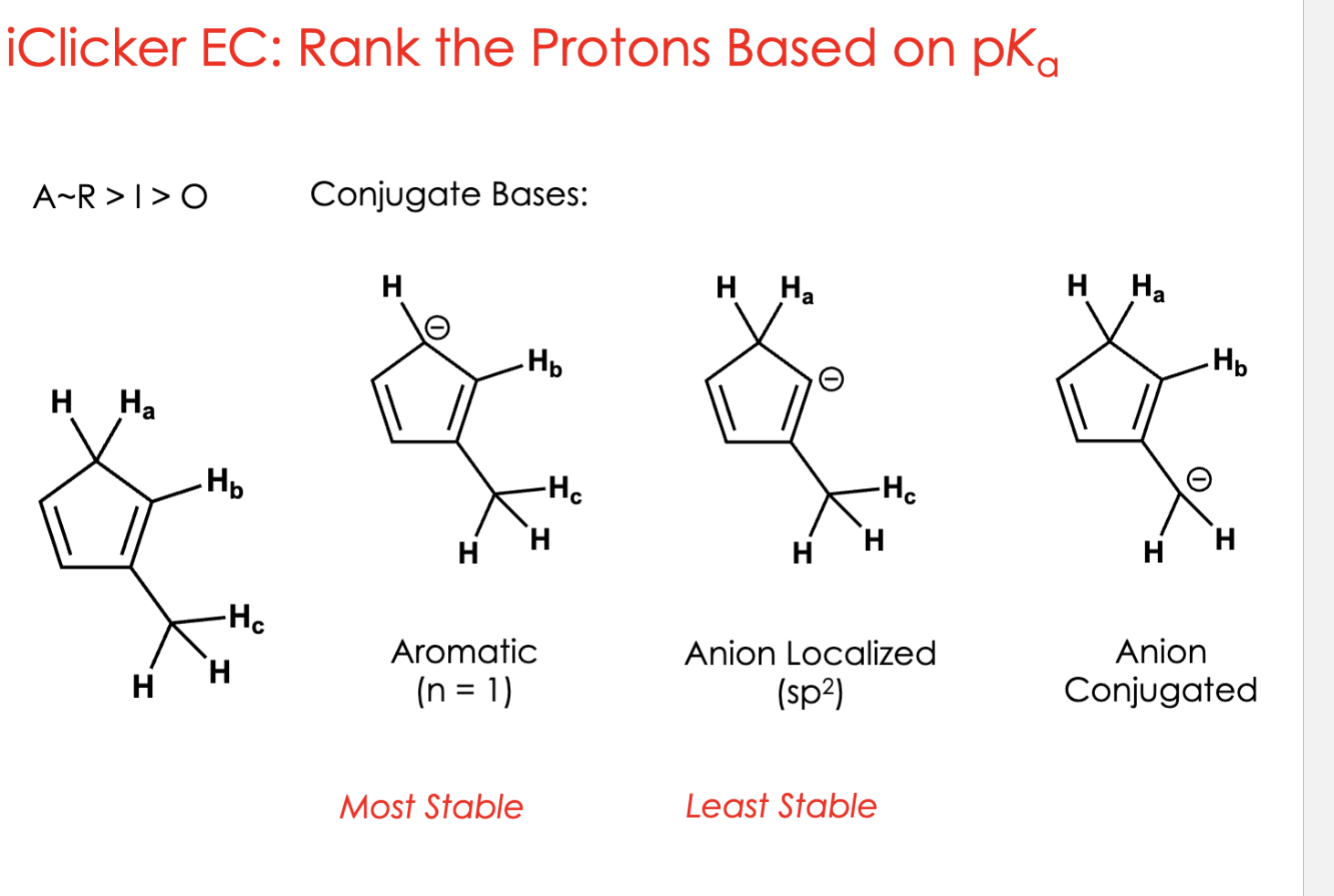
Ha < Hc < Hb
The more acidic a hydrogen is, the easier it comes off (gives away its proton).
That means → the negative charge left behind (the conjugate base) is more stable.
So we’re asking:
Which hydrogen’s conjugate base is most stable?
🧠 Step 2: The pattern to remember
👉 More stable conjugate base = stronger acid = lower pKa
So the lowest pKa means the most acidic hydrogen.
Ha
When you remove Ha, the leftover negative charge can move around the ring through resonance.
This makes the conjugate base aromatic (super stable).
✅ Delocalized charge = very stable
✅ Aromatic = extra stable
→ So Ha gives the most stable conjugate base → strongest acid (lowest pKa)Hb
When you remove Hb, the negative charge is stuck on one atom (localized).
No resonance = can’t move around = less stable.
❌ Localized charge = less stable
→ So Hb is the least acidic (highest pKa).
Hc
When you remove Hc, the negative charge is partially shared with the ring.
It can move a little, but not as much as Ha.
⚡ Some delocalization (resonance), but not aromatic
→ So Hc is in the middle — more acidic than Hb, but less than Ha.
What are non-covalent interactions?
Attractive or repulsive forces between atoms or molecules that are not covalent bonds.
They help molecules stick, fold, and interact, but they’re weaker than covalent bonds.
What are intermolecular interactions?
between different molecules
What are intramolecular interactions?
within the same molecule
What’s a boiling point?
It’s the temperature where a liquid turns into a gas 🌡💨
That happens when the liquid’s vapor pressure = the air pressure around it.
What’s the relationship between IMF and boiling point
Molecules in a liquid are held together by IMFs (non-covalent forces).
To boil, you have to pull them apart — that takes energy (heat) 🔥.
The stronger the IMFs → the harder it is to separate them →
➜ you need more heat → higher boiling point.
Stronger IMF = ?
Lower IMF = ?
Stronger IMF = Higher Boiling Point (BP)
Weaker IMF = Lower Boiling Point (BP)
Stronger IMF: hydrogen bonding
Weaker IMF: london dispersion
gimme the strength of non covalent interactions (lower to increasing)
London dispersion forces
Dipole dipole forces
Hydrogen bonding
Covalent bonding
Ionic bonding
FILL OUT THE TABLE: COVALENT IONIC
TYPE:
WHO’s INVOLVED:
WHAT HAPPENS TO ELECTRONS:
STRENGTH:
TYPE: COVALENT
WHO’s INVOLVED: NM + NM (RIGHT SIDE)
WHAT HAPPENS TO ELECTRONS: Shared
STRENGTH: SRONG
TYPE: IONIC
WHO’s INVOLVED: METALS (L) + NM
WHAT HAPPENS TO ELECTRONS: Transferred
STRENGTH: STRONG BUT BREAKS IN WATER
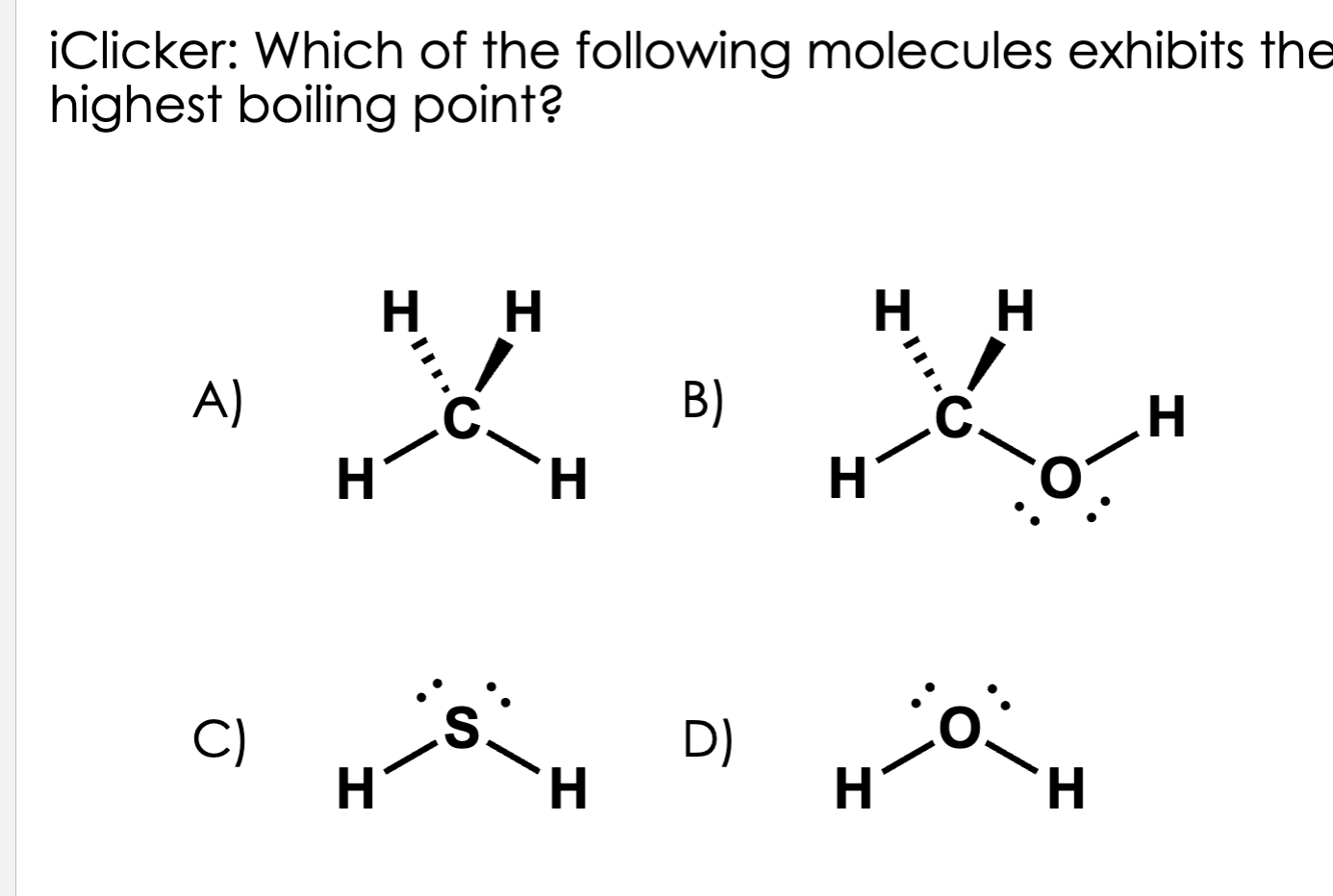
D
🧲 Step 1: What holds molecules together?
Every molecule has intermolecular forces—that’s what keeps them close in a liquid.
There are 3 main kinds (from weakest to strongest):
1⃣ London Dispersion Forces (LDF) — tiny temporary attractions (every molecule has them)
2⃣ Dipole-Dipole Forces — between molecules with positive and negative ends
3⃣ Hydrogen Bonds — special, super-strong dipole forces with H bonded to N, O, or F
So:
Stronger force → molecules stick tighter → harder to pull apart → higher boiling point
⚗ Step 2: Look at each molecule
Molecule | Name | Type of IMF | Why |
|---|---|---|---|
A) CH₄ | Methane | Only LDF (weakest) | Nonpolar, no charges |
B) CH₃OH | Methanol | Hydrogen bonding | Has O–H bond |
C) H₂S | Hydrogen sulfide | Weak dipole only | S is not electronegative enough for real H-bonds |
D) H₂O | Water | STRONG hydrogen bonding | Each molecule can form 4 H-bonds (2 from H’s, 2 from lone pairs) |
✅ Step 4: How to tell next time
If you’re ever lost, ask yourself:
1⃣ Does it have O–H, N–H, or F–H?
→ If yes, it can hydrogen bond (strongest).
2⃣ Is it polar (has +/– ends)?
→ Dipole–dipole (medium).
3⃣ If not, only dispersion forces (weakest).
Then rank boiling points by force strength:
Hydrogen bonding > Dipole–Dipole > Dispersion
If a molecule has more than one force → how do we decide
the strongest one wins
🧩 Q1: Which type of force is the strongest overall?
A) Dipole-dipole
B) Hydrogen bonding
C) Ionic or covalent
D) London dispersion
WHY?
✅ Answer: C) Ionic or covalent
💬 Explanation:
These are not between molecules — they hold atoms together inside a molecule, so they’re way stronger than the forces between molecules.
🧩 Q2: What’s the strongest intermolecular force?
A) Hydrogen bonding
B) Dipole–dipole
C) London dispersion
WHY?
✅ Answer: A) Hydrogen bonding
H-bonds happen only with O–H, N–H, or F–H and are much stronger because those atoms are small and very electronegative — they pull really hard on H.
Solubility: define
When you mix two substances, you want to know if one will disappear into the other — that’s solubility.
Whats the key idea with solubility:
✅ Polar dissolves polar
✅ Nonpolar dissolves nonpolar
🚫 Polar does not dissolve nonpolar (they separate like oil and water)
Solute vs solvent
Solute: the thing being dissolved
Solvent: the thing doing the dissolving
Miscible vs. Immiscible
Miscible
Two liquids that mix completely
No layers form — they look like one liquid
✅ Example: Water + ethanol → mix perfectly (both polar)
🛢 Immiscible
Two liquids that don’t mix
They separate into layers
🚫 Example: Water + oil → don’t mix (one polar, one nonpolar)
💬 Think: “Immiscible = Impossible to mix”