Chapter 10: Covalent Bonds
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Atoms Share Electrons to Form Molecules
• Ionic compounds are formed by transfer of electrons between
metal and non metals.
• Covalent compounds are formed by sharing of electrons between
non metal atoms.
• Atoms gain, lose, or share electrons to achieve a stable, noble-gas
electron configuration, ns 2np6.
Octet Rule and Exceptions
general trend to achieve 8 valence electrons
Exceptions:
Odd Electron Molecules / Free Radials
Electron Deficient Molecules
Hypervalent molecules / Expanded Octet Molecules
Duet Rule
Hydrogen, which has valence electrons in the first shell, 1s², follows this rule
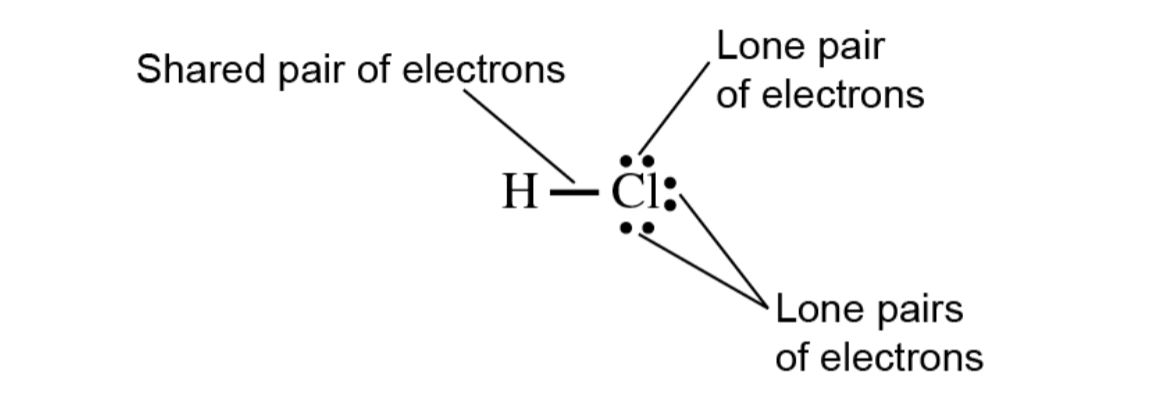
Figure 10.2 Lewis Structure of HCl
H atom has 1 valence electron and a Cl atom has 7
In HCl molecule, these 2 atoms share 1 pare of electrons
H has access to asecond electron (duet rule) via the shared pair
Cl has three unshared pairs, lone pairs, and 1 shared pair, forming a covalent bond
Total # of valence electrons on molecule
5e- + 3(1e-) =8e-
Lewis Structure Example: NH3
First Step: Find the number of valence electrons
Step 2: Find out the central atom (the most electronegative one= more protons)
Step 3: Draw the Skeleton Structure (1 bond = 2 electrons) NH3 has 3 H bonds = 6 electrons
Step 4: 2 electrons are left, so put them on the N atom
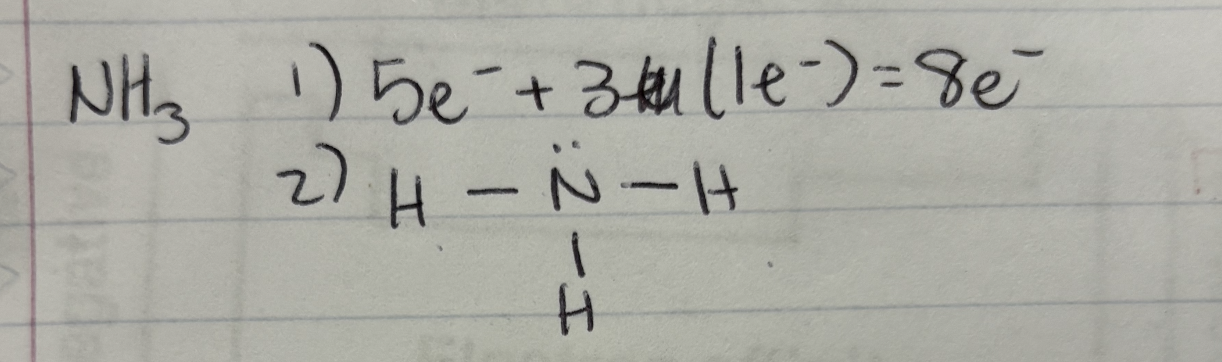
Lewis Structure Example: Methanol
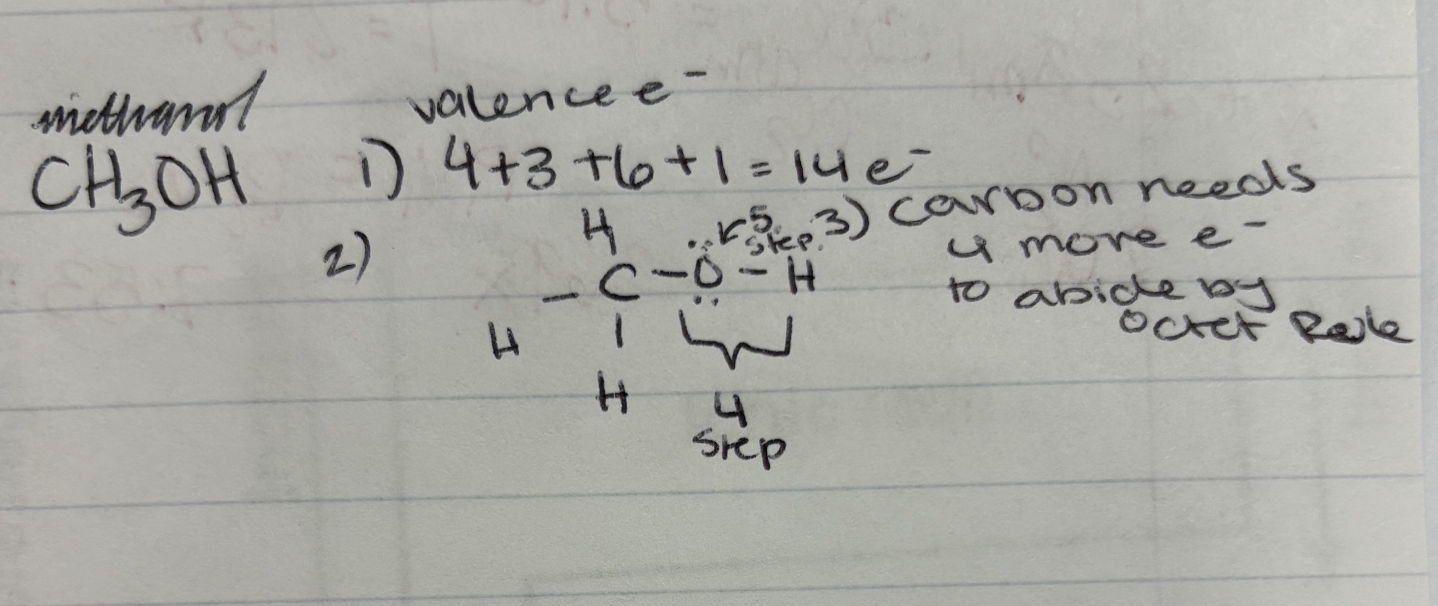
Lewis STructures of Polyatomic. Ions
Polyatomic ions consist of two or more atoms covalently bonded
together that have a net positive or negative charge.
• Place the structure within brackets, with the charge indicated
outside the brackets.
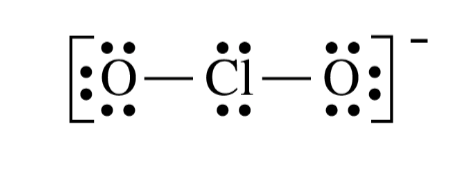
Odd Electron Molecules: Free Radicals
• Molecules with an odd number of valence electrons typically have
one atom with seven valence electrons.
• Molecules with single unpaired electrons are referred to as
radicals.
• Common examples are NO (11 total valence electrons) and NO2
Electronegativity
the tendency of a bonded atom to attract shared electrons to itself
Higher EN = Stronger attraction to electrons
General trend: EN increases
L to R across a row of PT
Bottom to Top within a periodic table group
Lighter noble gases don’t have defined EN values becausae they form no bonds
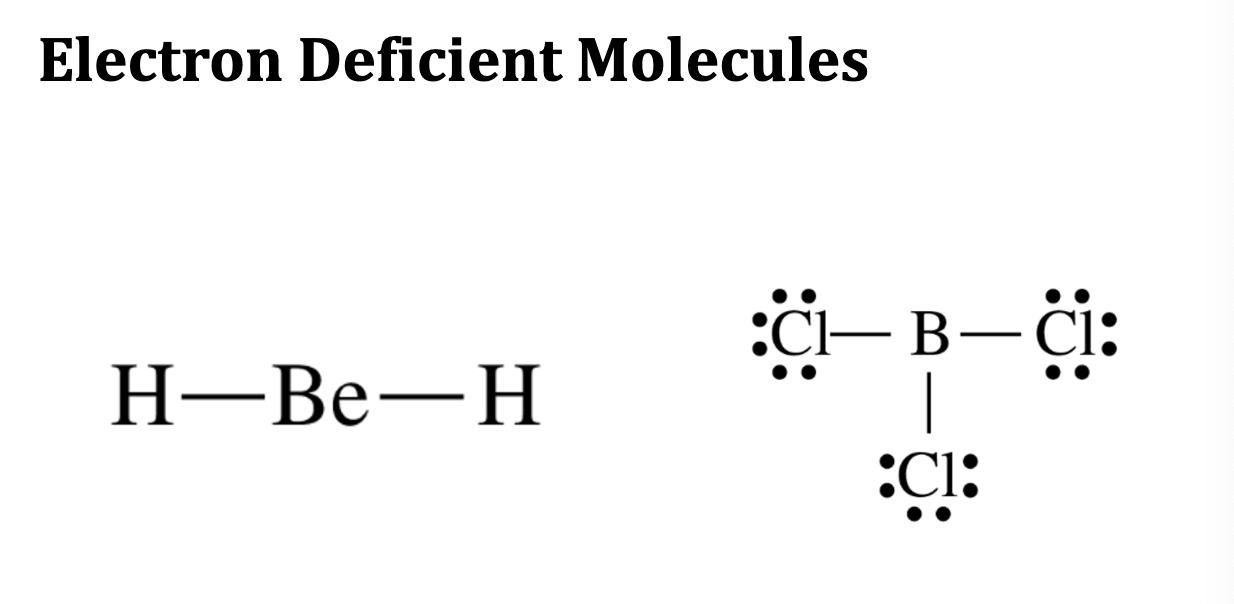
Electron Deficient Molecules
Hydrogen forms a duet, not an octet.
• Other very small atoms also have form molecules with less than an
octet.
• Beryllium forms molecules with four electrons in its valence
shell.
• Boron atoms, in molecules, generally have six electrons in their
valence shells.
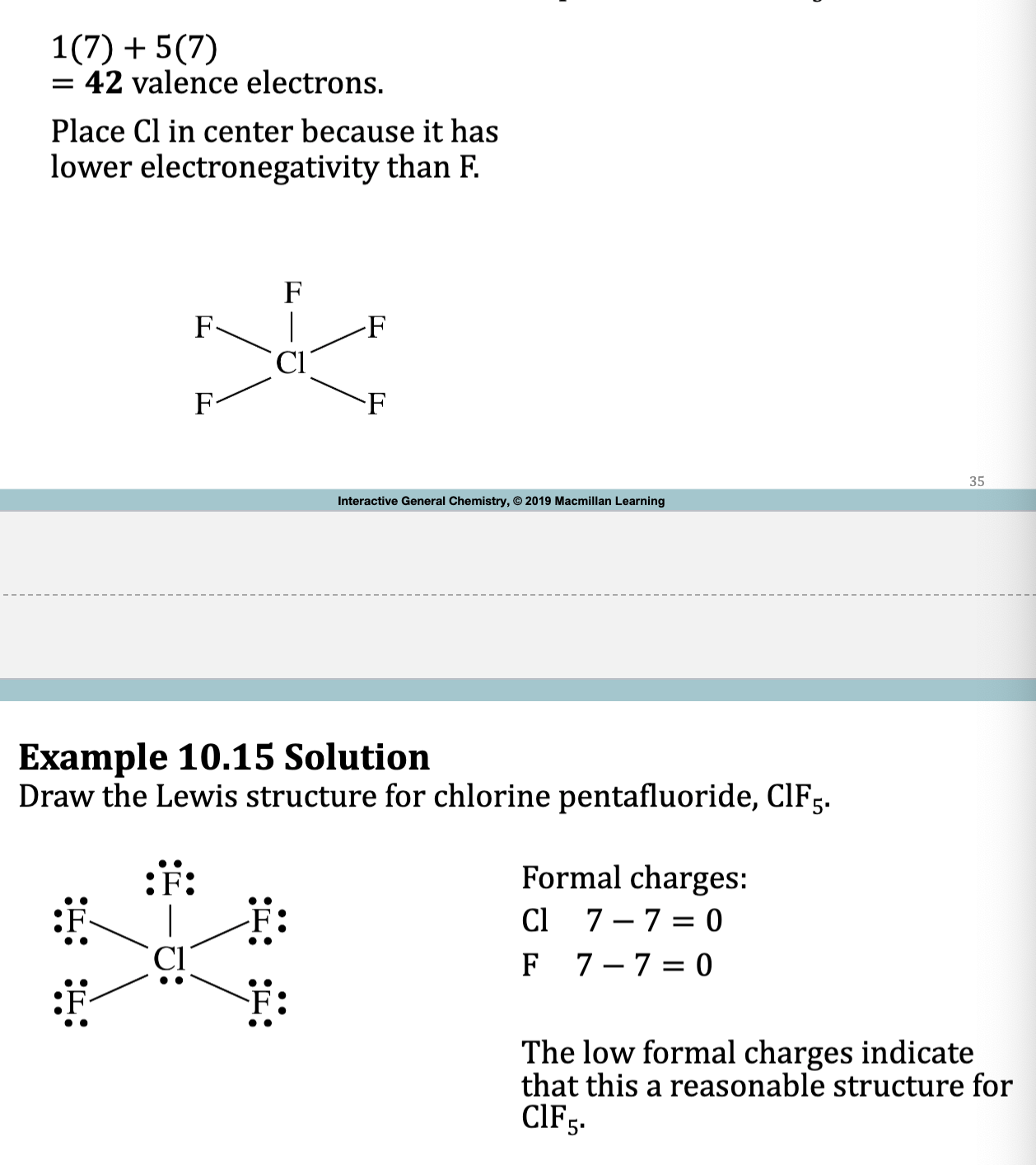
Hypervalent Molecules / Expanded Octet Molecules
• If the central element in a molecule or polyatomic ion is in the
third period or beyond, period 3,4 5 etc, it can sometimes share
more than four pairs of electrons.
• Expanded octets result in lower formal charges, in most cases.
Example 10.15: Draw the Lewis Structure for chlorine pentafluoride, ClF5
Resonance
When a single Lewis Structure Cant properly describe a molecule or ion:
double bonds/lone pairs are able to jum around
overall charge
full octet cant be achieved without moving bonds around (double/triple bonds)
• Molecules/ions that have such structures (structures that differ
only in the placement of multiple bonds and lone pairs)are said to
exhibit resonance and the structures are referred to as
resonance structures
if a resonance structure is good, it is because the formal charge on all atoms have been minimized
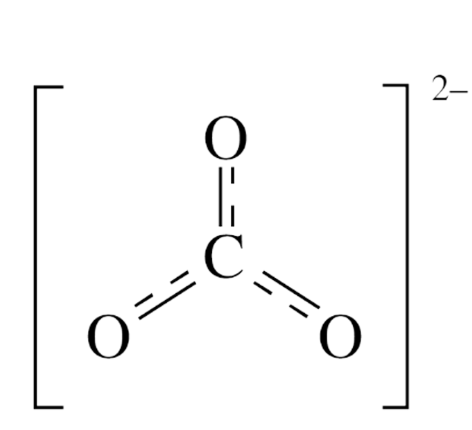
Interpreting Resonance Structures
• It is equally likely that the double bond is located between C and
any one of the three O atoms.
• In fact, the pair of electrons shown as the double bond is shared
(spread out) among all three locations.
• This is a delocalized bond and is designated as such by the
double-headed arrows between the structures, or as a resonance
hybrid.
• All three C–O bonds in the carbonate ion are equivalent and have
properties in between a single and double bond.
Resonance Hybrid
This resonance hybrid
structure is an alternative way
of showing the delocalized
bond.
• All three C–O bonds in the
carbonate ion are equivalent
and have properties in
between those of a single and
double bond.
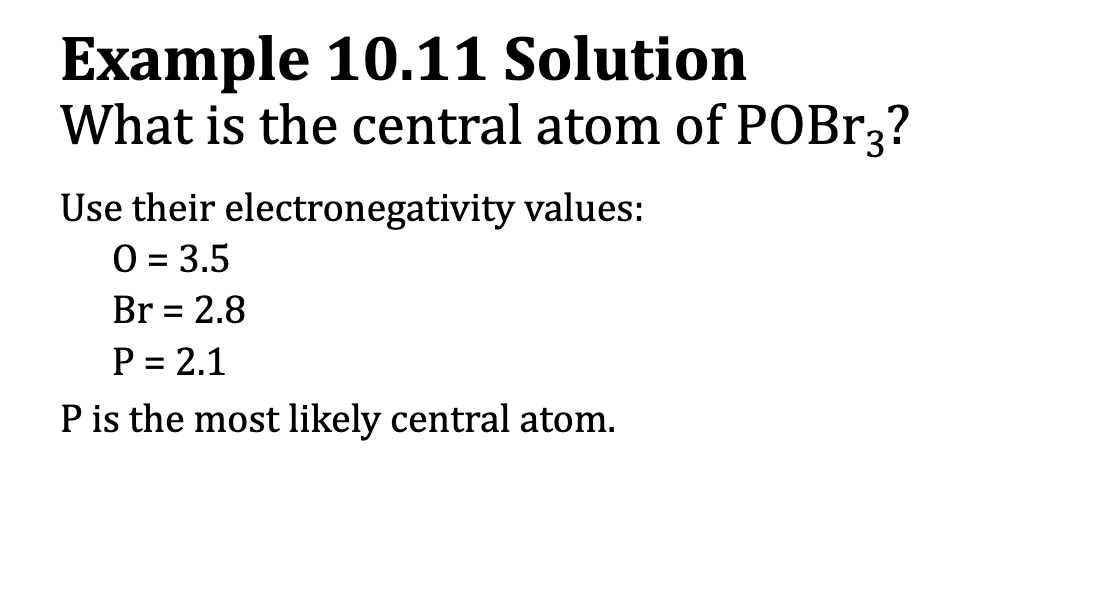
Electronegativity and the Central Atom
Elements with high electronegativity pull electrons closer to themselves when they share electrons, while elements with low electronegativity don't pull as strongly.
Because of this, highly electronegative elements are less likely to share electrons and are usually not the central atom in a molecule.
If two elements have the same number of valence electrons, the one with the lower electronegativity is usually chosen as the central atom.
E.x. 10.11: What is the central atom of POBr3?
Formal Charge
• Sometimes it is possible to draw multiple valid Lewis structures
that are not equivalent.
• We use concept of formal charge to decide which structure (or
structures) is the better representation of the real molecule.
• Formal charge is a type of electron bookkeeping in which you
assign a fictitious charge to each atom in a molecule
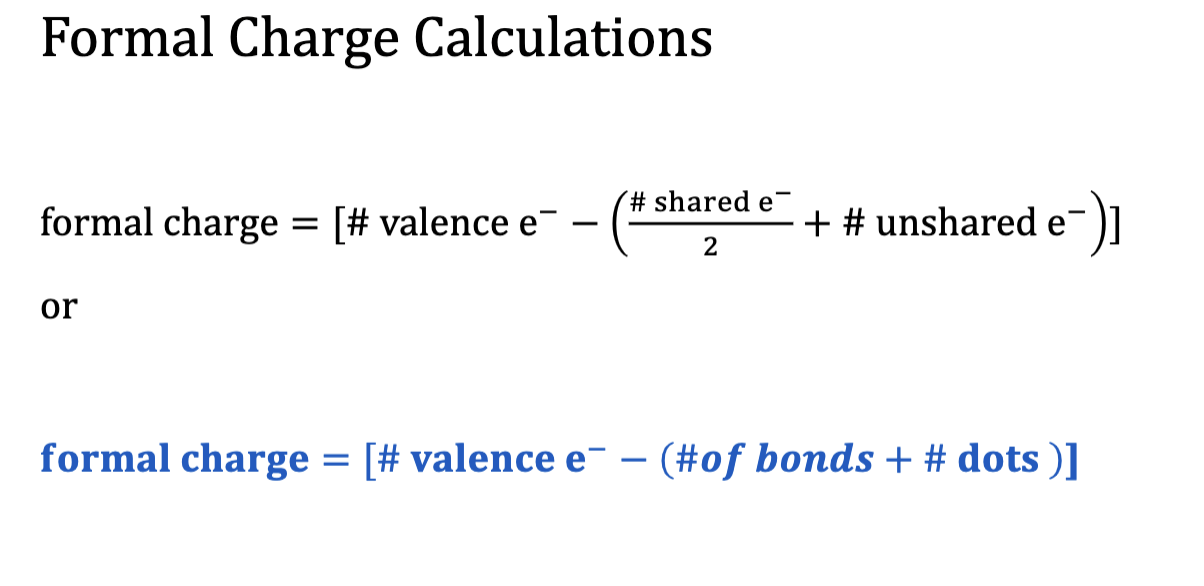
Formal Charge and Lewis Structures
• The most likely Lewis structures
• have small or zero formal charges, especially for the central atom;
• have negative formal charges associated with elements of
higher electronegativity; and
• have positive formal charges associated with elements of lower
electronegativity.
• Formal charge helps to determine which possible structure is more
energetically favorable
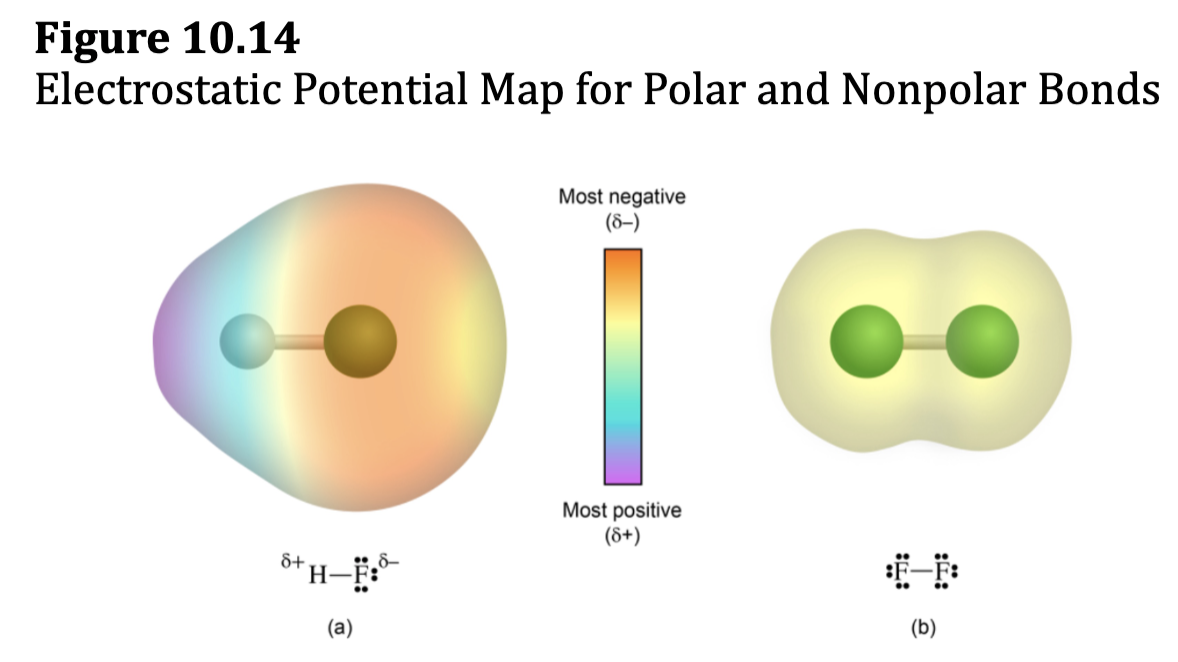
Electronegativity and Bond Polarity
• Two identical atoms share electrons completely evenly, but two
different atoms generally share electrons unevenly to form polar
covalent bonds.
• Hydrogen fluoride, HF
• The fluorine atom has a higher electronegativity than the
hydrogen atom.
• The charge cloud of the bonding electrons is more attracted to
F than H and, therefore, has a greater density around F than
around H
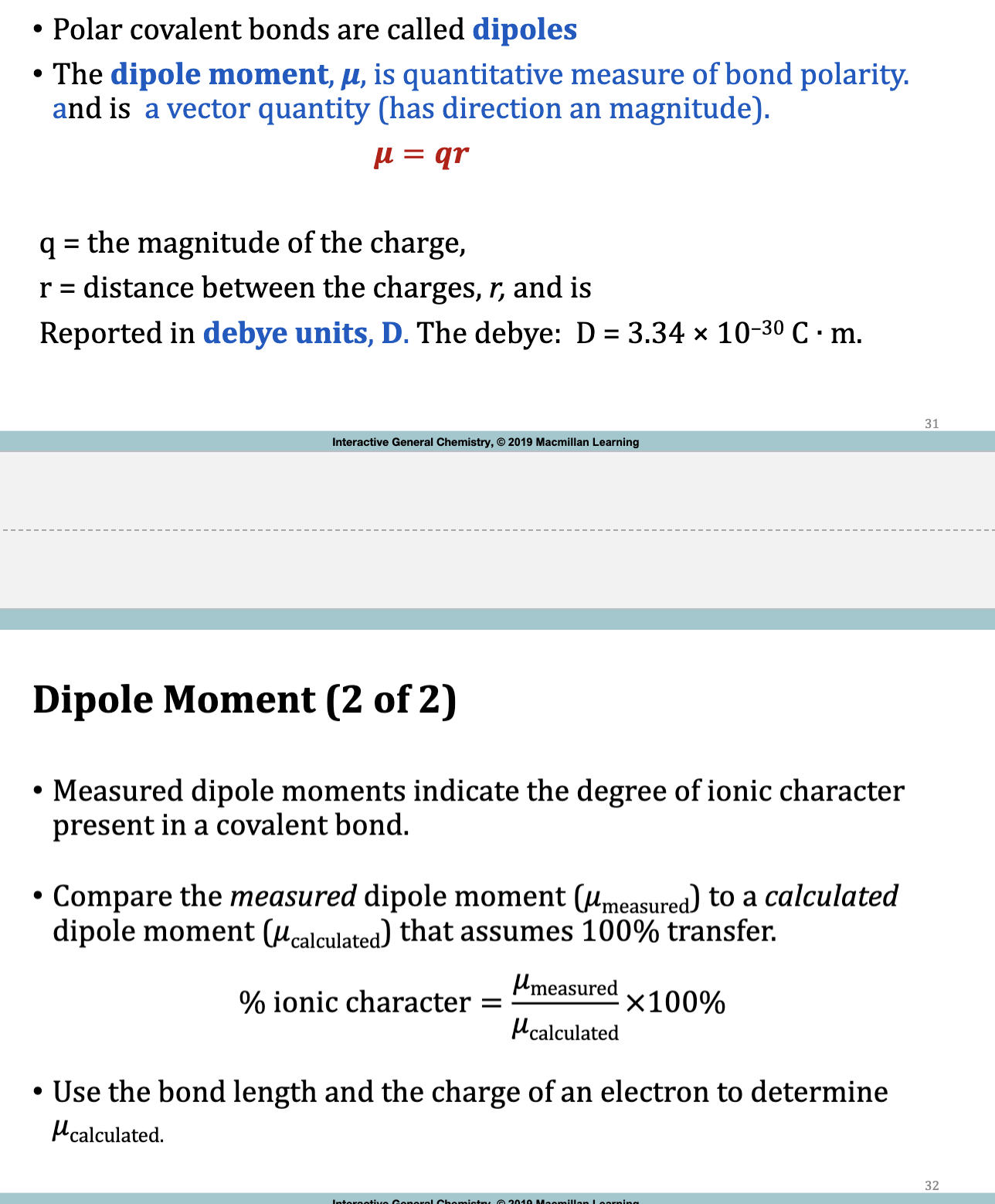
Dipole Moment
More Ionic Or More Covalent Bonds?:
K-F, N-N,Br-F, Cl-F
if 2 elements are close in proximity to each other on PT they have similar electronegativities as they get further apart the difference increases
- large electronegativity difference = ionic bond
small electronegativity diff = polar covalent bond
no difference in electronegativities = nonpolar covalent bond
The ___ the electronegativity difference, the ____ the dipole moment
larger; greater
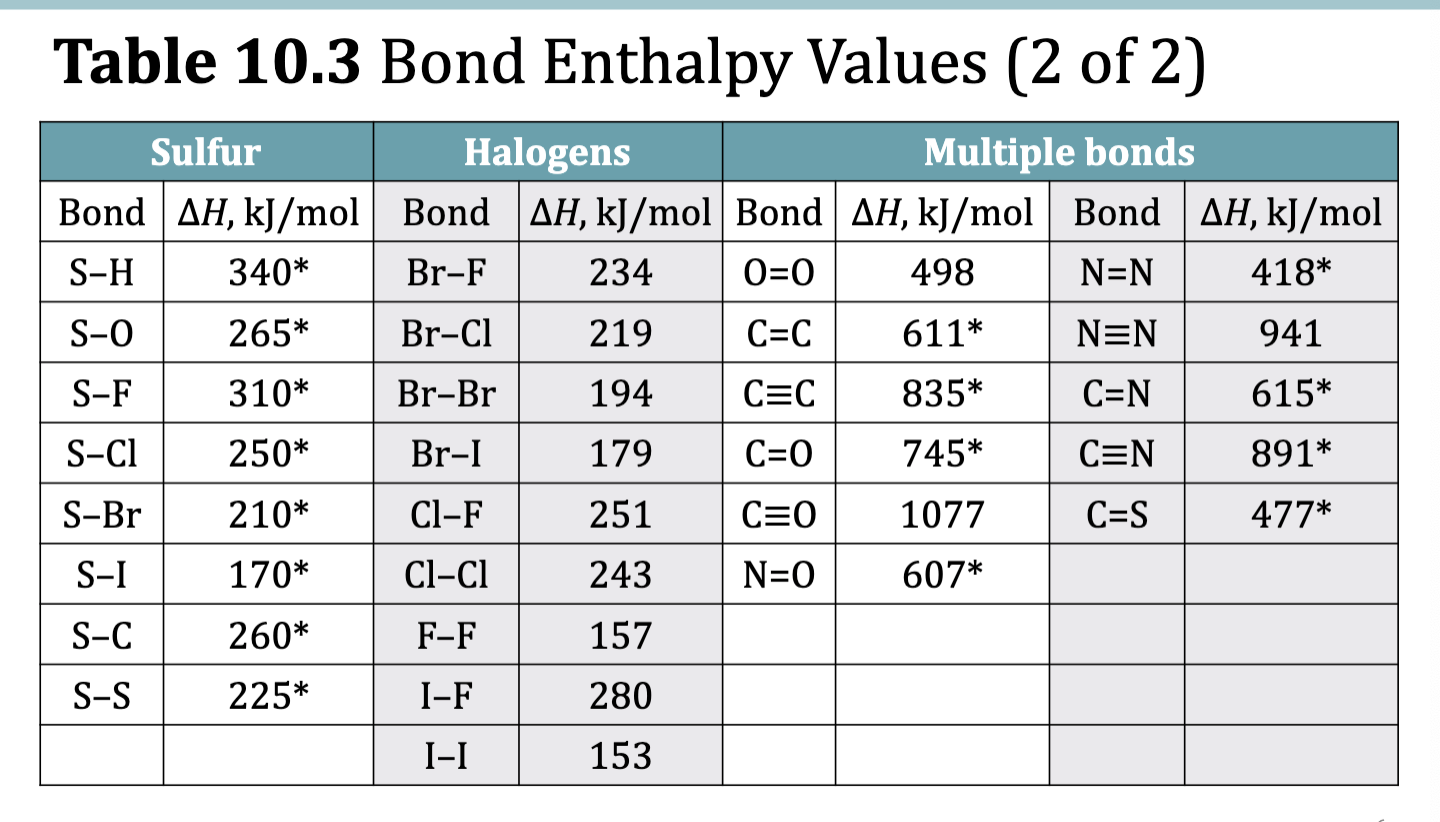
Bond Enthalpy: Bond Dissociation Energy
The strength of the bonds within a molecule determines the
stability of that molecule.
• Bond strength is measured as bond enthalpy, the enthalpy change associated when breaking a specific bond in 1 mol of gaseous molecules
exact bond enthalpies can be measured for diatomic elements
most bond enthakpy values are average values obtained from measurements from many different molecules containing a specific bond
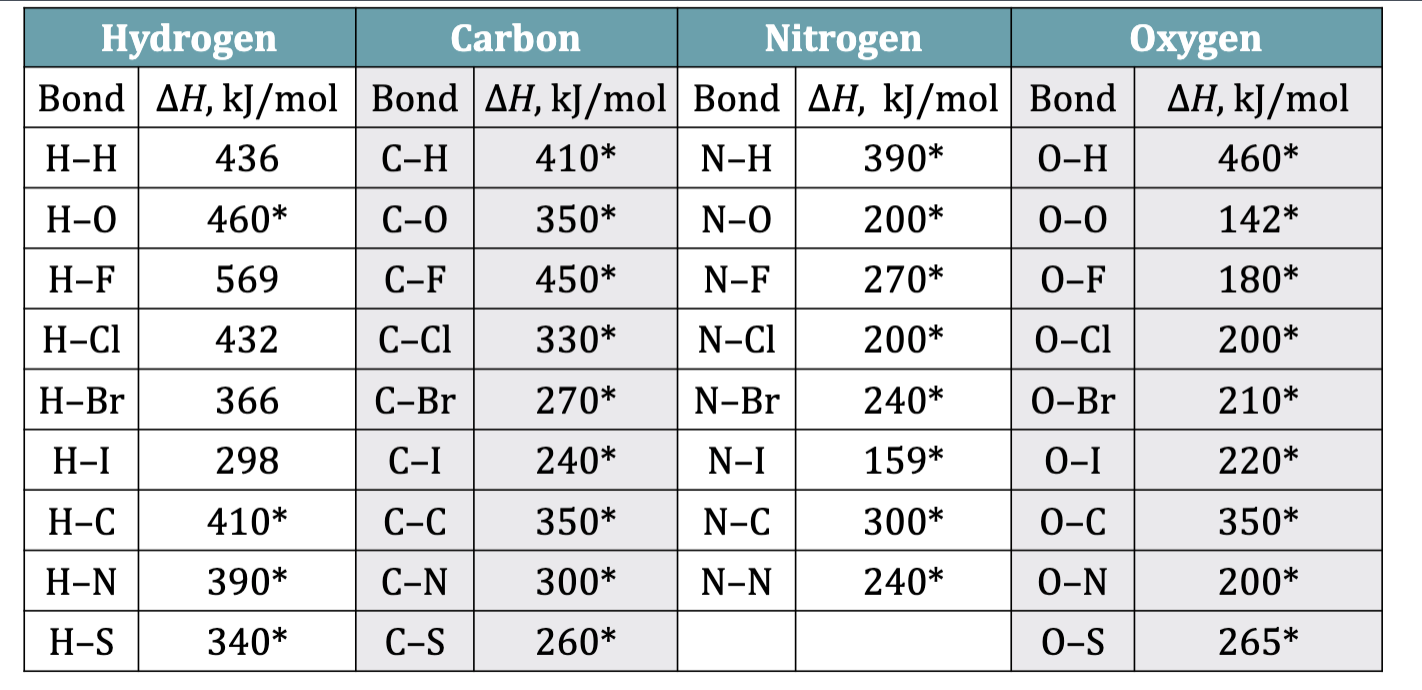
Table 10.3 Bond Enthalpy Values
Sample Calculation: deltaH for the formation of H2O from its elements H2 and O2
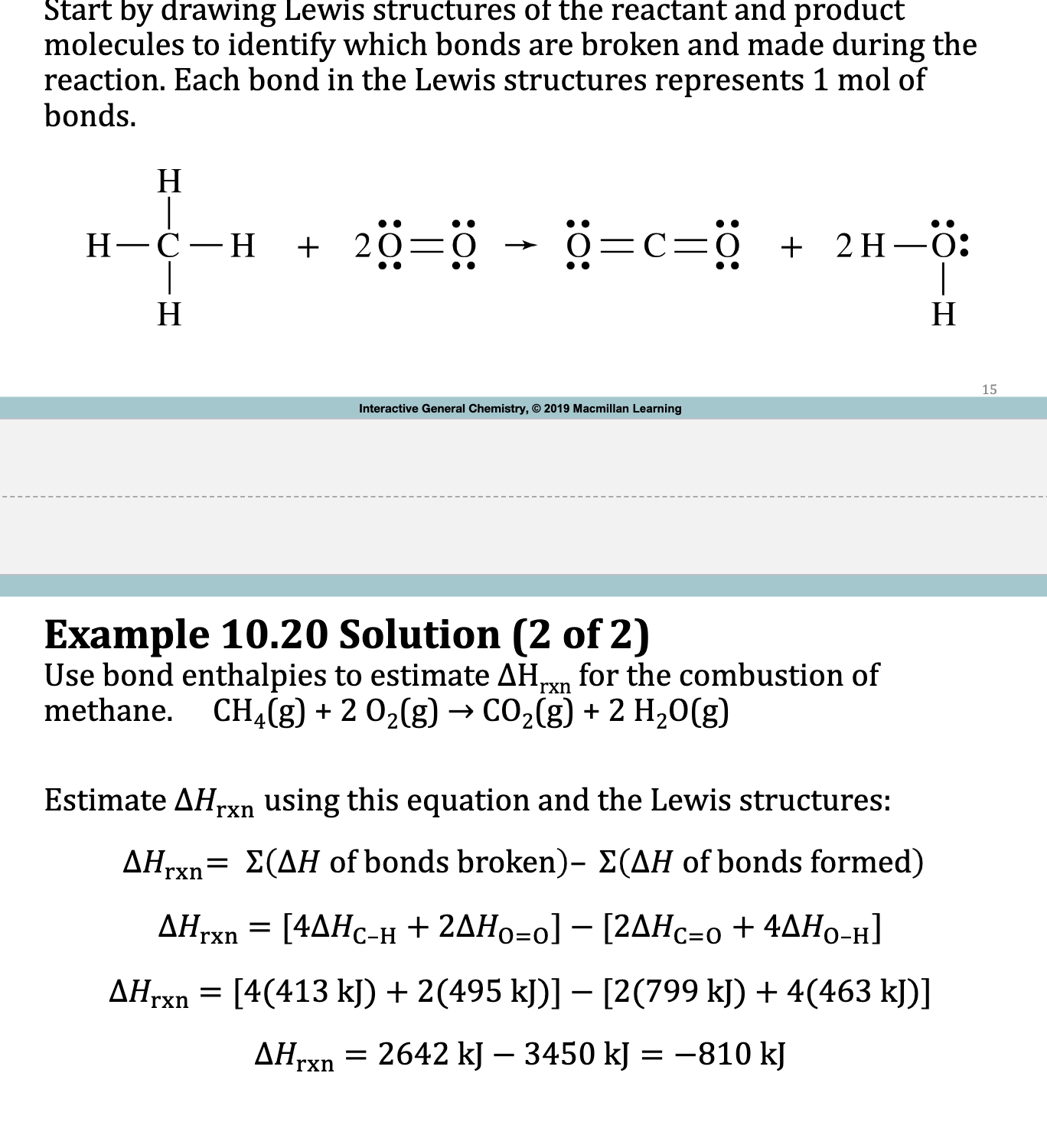
Do Lewis Structure of rxn
Refer to Table 10.3 and incorporate it into rxn and use formula
[2(436)+(498)] - [4(460)]
4 is coeff for products because there are 2 OH bonds and there is a coefficient of 2 in front of H2O So you multiply
![<ol><li><p>Do Lewis Structure of rxn</p></li><li><p>Refer to Table 10.3 and incorporate it into rxn and use formula</p><ol><li><p>[2(436)+(498)] - [4(460)]</p><ol><li><p>4 is coeff for products because there are 2 OH bonds and there is a coefficient of 2 in front of H<sub>2</sub>O So you multiply</p></li></ol></li></ol></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b6172825-ee2d-424b-9c4b-9c240467c36d.png)
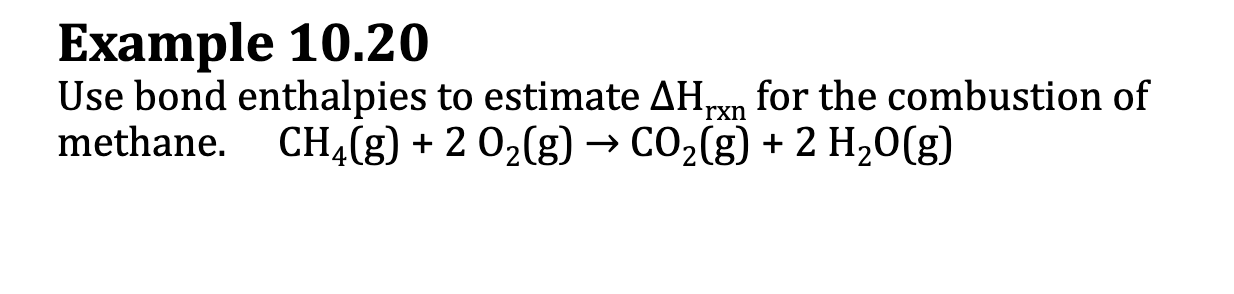
E.x. 10.20
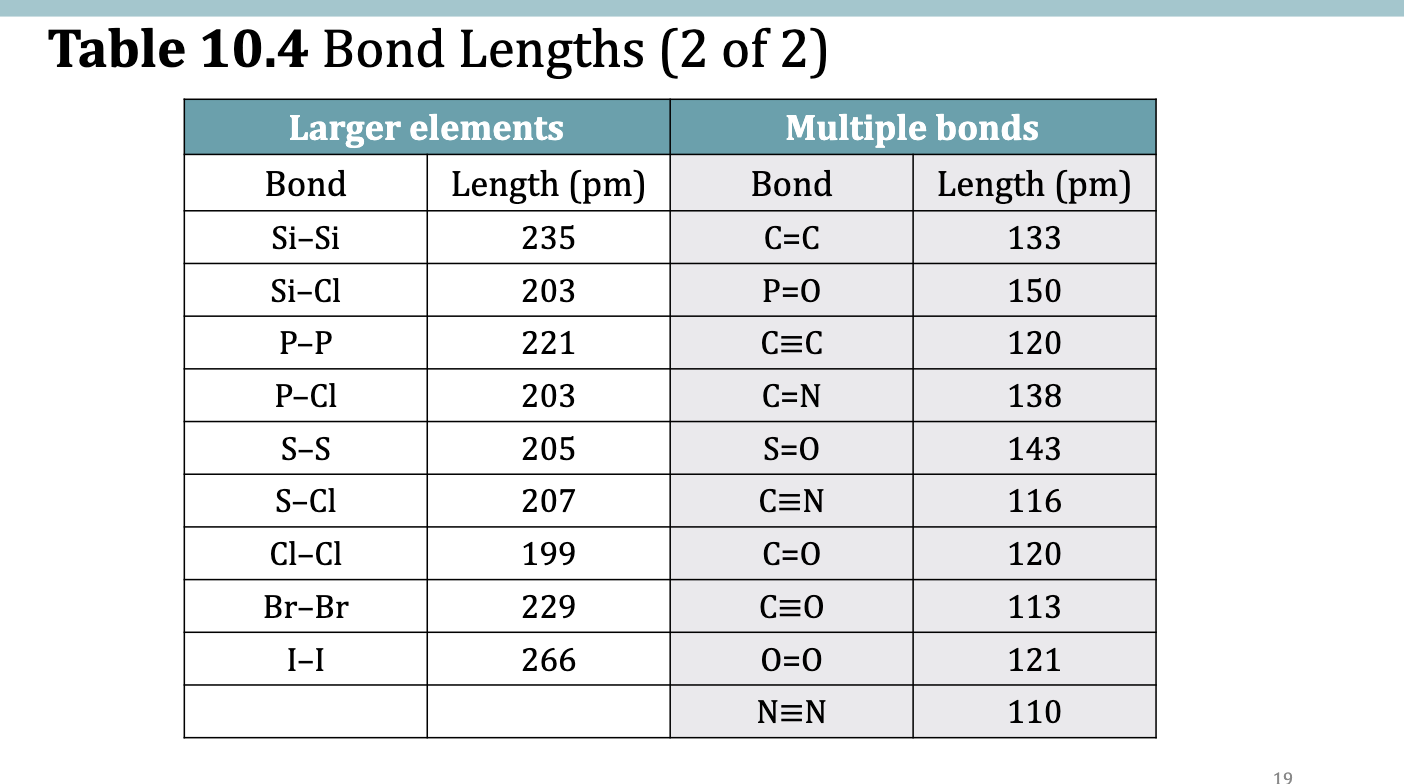
bond length
the distance between the nuclei of 2 atoms that share electrons in a covalent bond
BL increases as Bond enthalpy decreases
Longer bonds tend to be weaker bonds
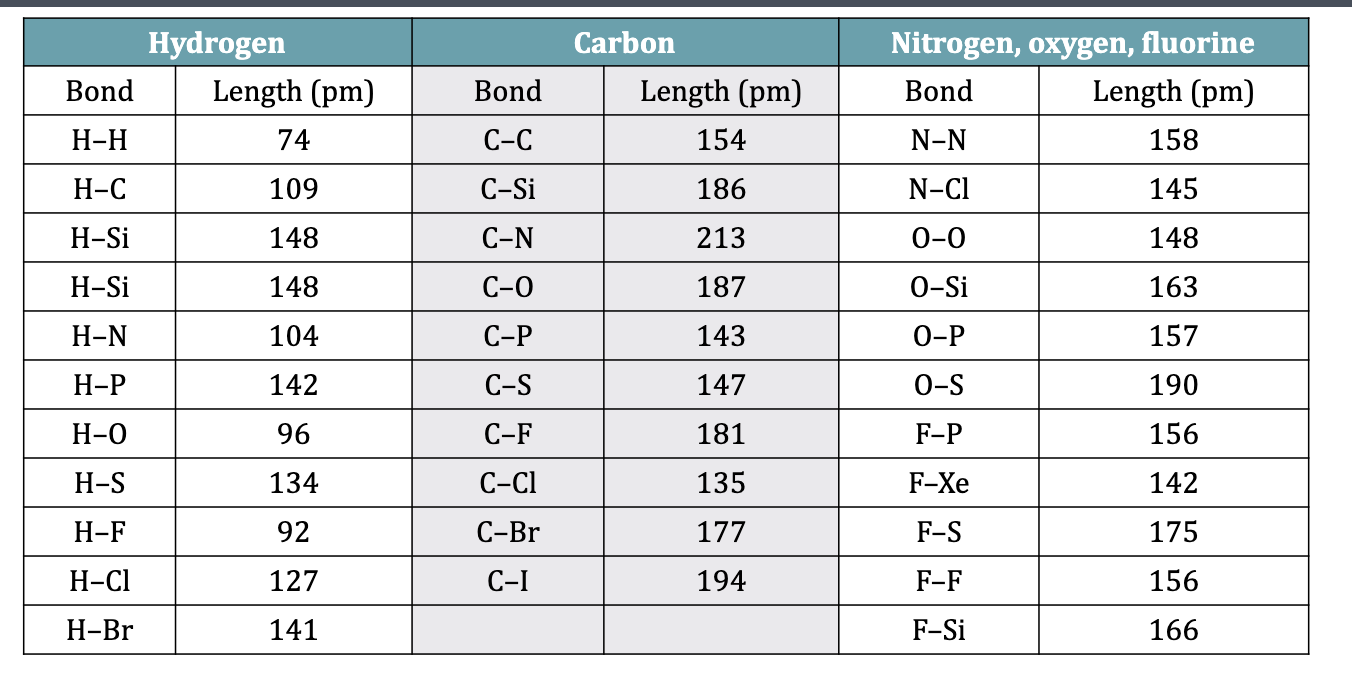
Table 10.4 Bond Lengths
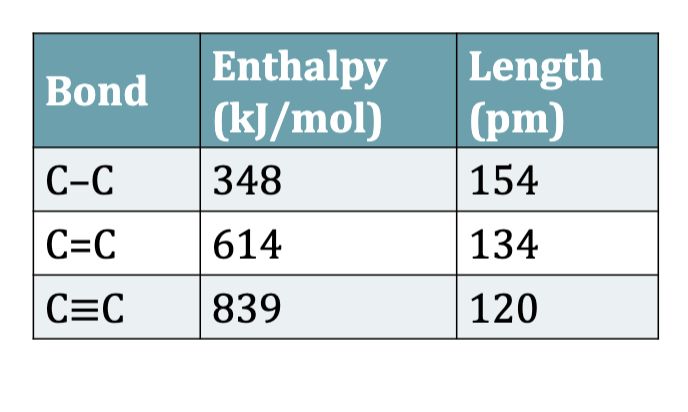
Bond Lengths and Bond Enthalpies
As bond length decreases from
single bond to double bond to
triple bond, bond enthalpy
increases. Shorter bonds are
stronger bonds
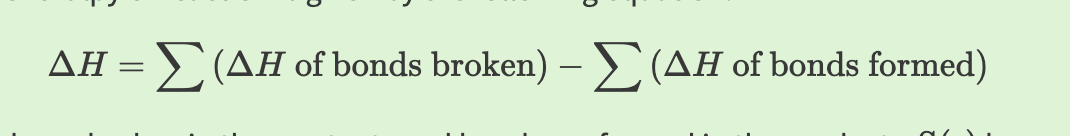
Enthalpy of Reaction Formula
reactants - products
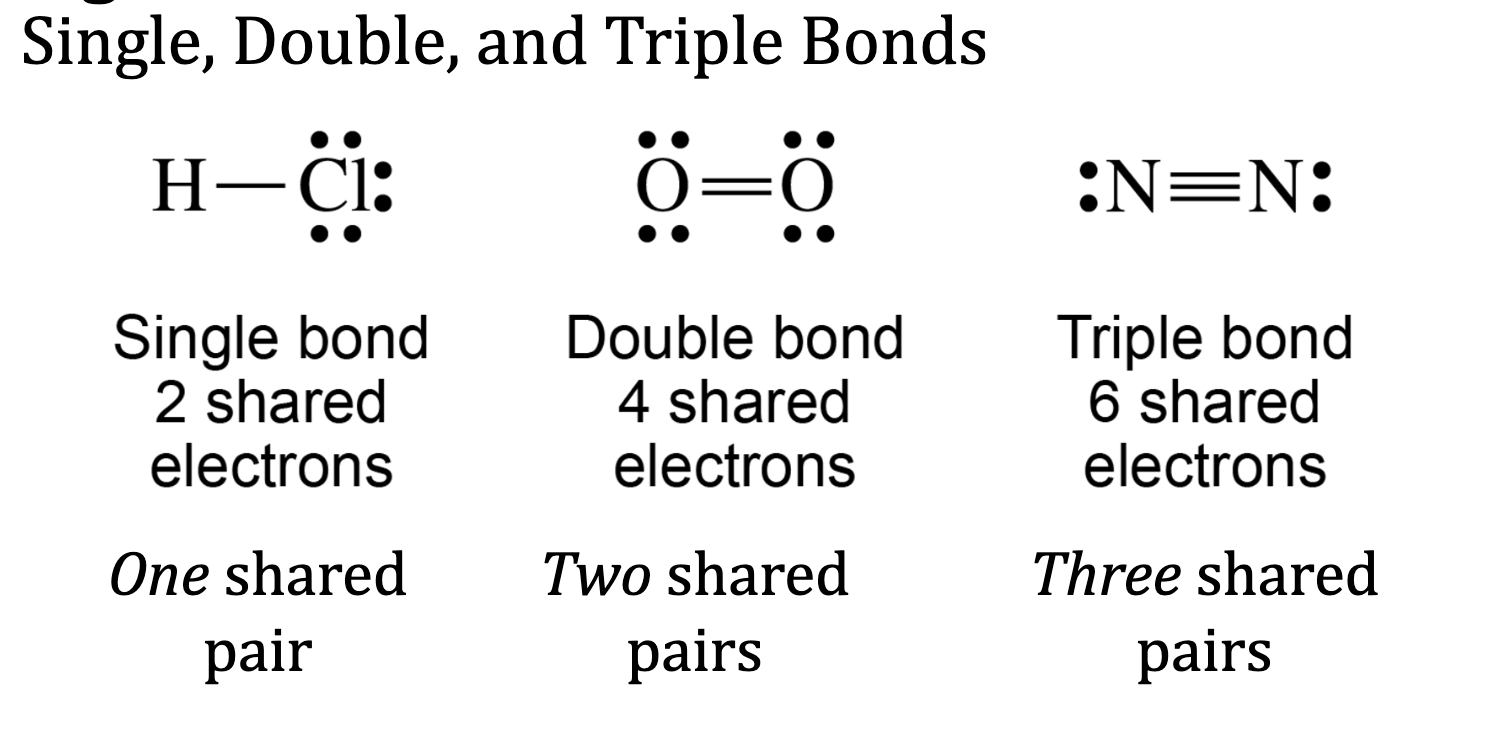
When do i Double Bond?
central atom needs more bonds to follow octet rule (if C has 3 single bonds and an atom next to it has lone pairs)
Some atoms ❤ bonding: C, N (triple and double), O, S, and P (esp in expanded octets)
DO NOT double bond H, or Halogens (1 bond and 3 pairs is preferred
Bond Strengths
breaking strong bonds requires energy = endothermic
forming strong bonds releases energy = exothermic