Cells alive 7 - Stem cells
1/18
Earn XP
Description and Tags
Define stem cells and describe their key characteristics. • Identify and distinguish the major types of stem cells. • Explain the concept of the stem cell niche and describe where they are found in the body. • Describe the pre-clinical and clinical relevance of stem cells in veterinary medicine
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
what are stem cells?
cells that have the ability to replicate themselves: self-renewal
capable of differentiating into many different cell types
Importance:
support tissue growth during developmental stage
maintain tissue homeostasis: replace cells that are lost or naturally turn over
repair and regenerate after injury
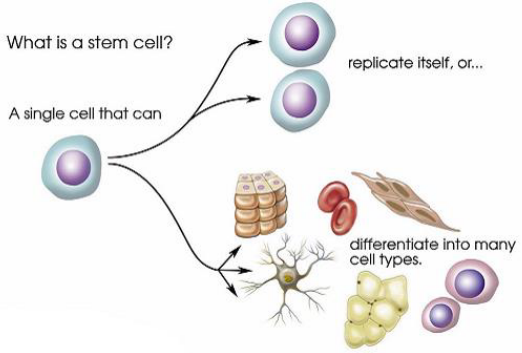
what are the stem cell types?
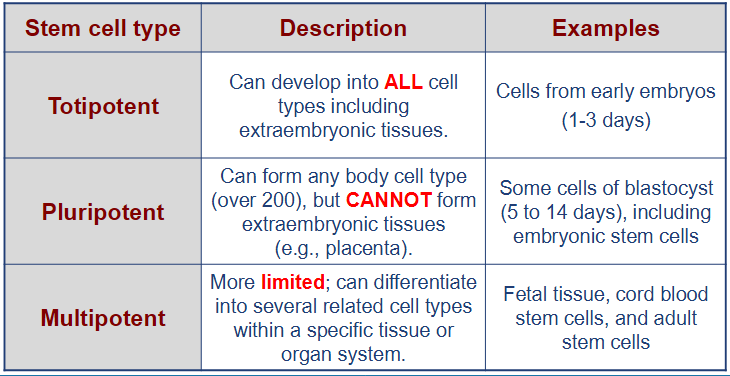
what are totipotent cells?
can develop into ALL cell types including extraembryonic tissues
cells from early embyos (1-3 days)
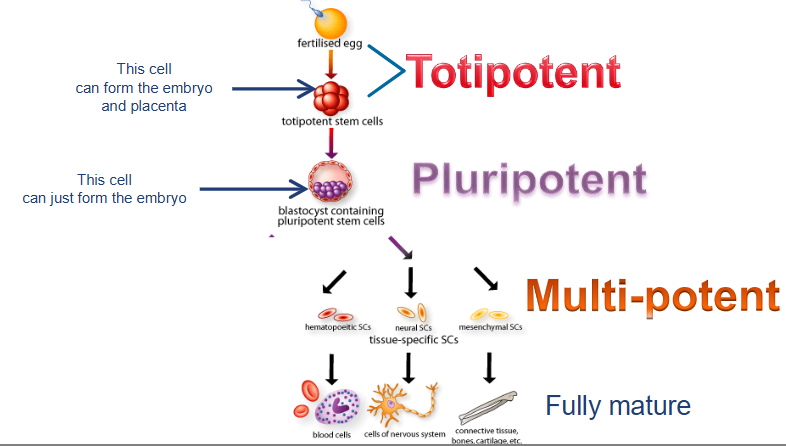
what are pluripotent cells?
can form any body cell type (over 200) but CANNOT for extraembryonic tissues (e.g. placenta)
some cells of blastocyst (5 to 14 days) including embryonic stem cells
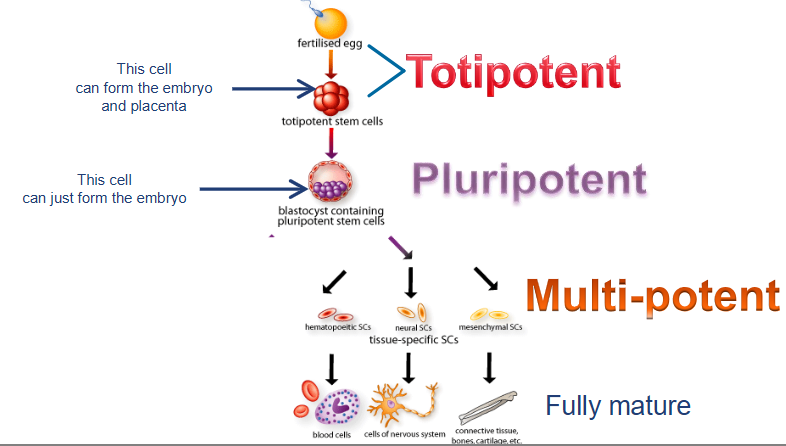
what are multipotent cells?
more LIMITED; can differentiate into several related cell types within a specific tissue or organ system
fetal tissue, cord blood stem cells, and adult stem cells
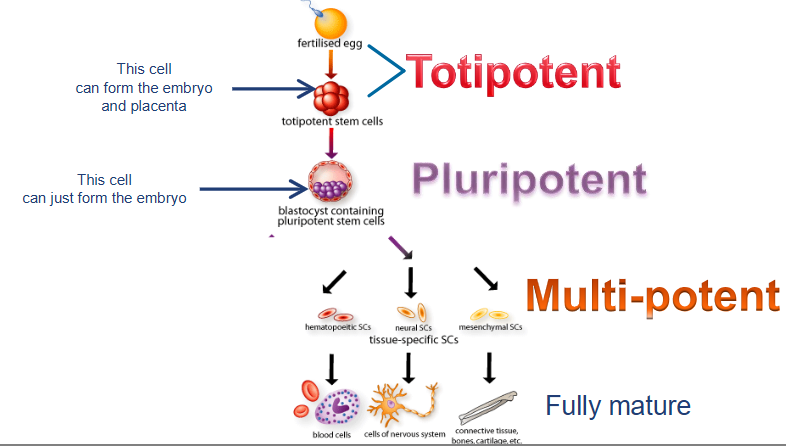
how many types of stem cells exist?
once the cells are fully differentiated they are called UNIPOTENT
they can still replicate (with limits) but they will give rise only to cells with identical phenotypic characteristics)
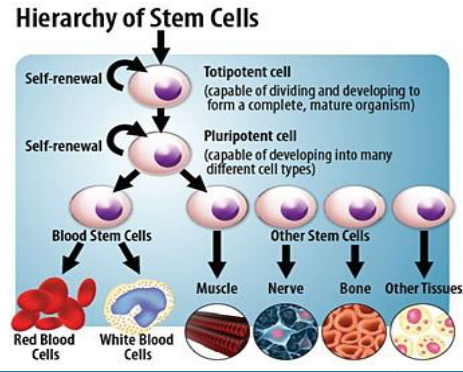
there are cells that cant’t be replaced - like what?
cardiomyocytes
photoreceptors - some lower vertebrates (e.g. zebrafish) can regenerate these cells
neurons - fail to replace completely new neurons (limited neurogenesis in hippocampus, olfactory bulb), fail to regenerate their axons (only in CNS; PNS), spinal cord injury, glaucoma, Alzheimer’s, Parkinson
hair cells inside cochlear

what is the stem cell niche?
Extrinsic microenvironment that supports and regulate stem cell behaviour within tissues
Stromal cells (not stem cells, just supporting cells):
-structural support
-growth factor production
-signalling molecules influencing the stemness of cells
Cell-to-cell interactions:
-contact between adult stem cells and neighbours to regulate cell fate
Cell-to-ECM (extracellular matrix) interactions:
physical and biochemical cues to guide proliferation and differentiation
Extrinsic microenvironment that supports and regulate stem cell behaviour within tissues
Functions:
-usually quiescent (dormant)
-cells usually kept at G0 unless stimuli are presesnt for proliferation and/or differentiation
-maintain stem cells self-renewal
-control differentiation
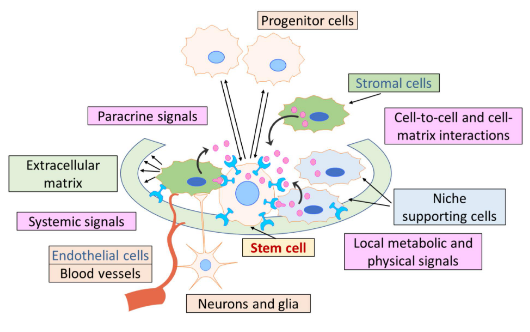
where can you find the stem cell niche?
bone marrow
heart (debatable)
skin
muscle
stem cell niche - bone marrow
dont need to know the details of the pics - same for the others below
just need to knw that stem cell found in bone marrow

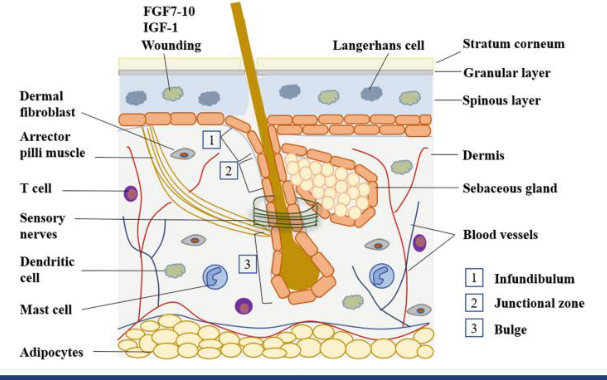
stem cell niche - skin
same for skin
but theres a specialised area in the skin, specially in the bulge, has stem cells
e.g. laser hair removal treatment - kiling the stem cells
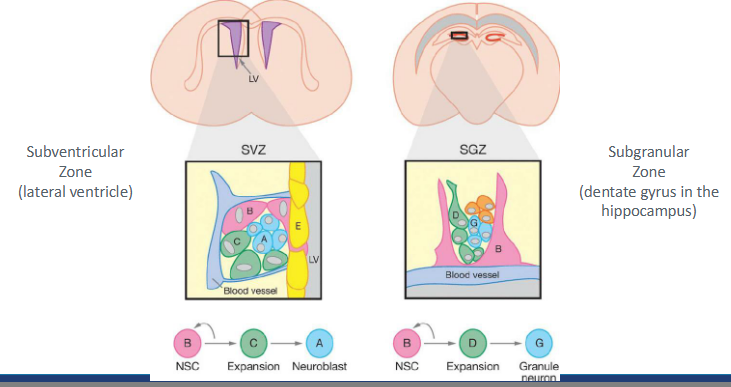
stem cell niche - CNS
same here
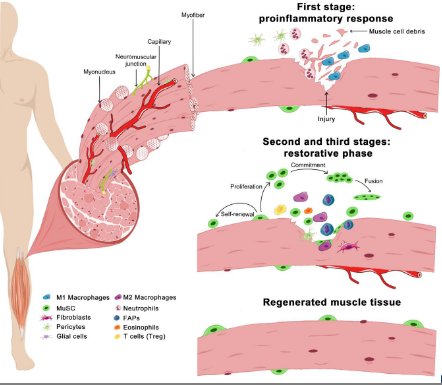
stem cell niche - muscle
image shows how stem cells act when muscle is injured
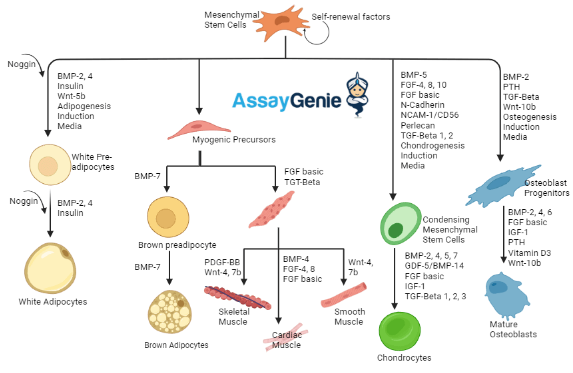
what are the signalling pathways that determine stem cell fate?
Major soluble factors:
• FGFs (fibroblast growth factors)
• EGF (epidermal growth factor)
• TGF-β
• IGFs (insulin growth factor)
Major pathways:
• BMP (bone morphogenetic protein)
• Wnt/β-catenin
dont need to know content in pic, its just helper
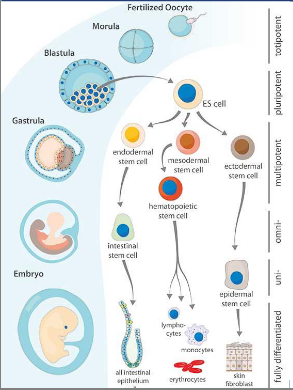
what are the embryonic stem cells?
• Derived from the inner cell mass of the early embryo (blastocyst)
• Pluripotent: can develop into ANY cell type in the body.
• Can be grown indefinitely in the lab if they are properly maintained
Clinical relevance:
• Cell transplantation: functional restoration
• Disease modelling: in vitro models for animal/human disease
what is induced pluripotent stem cells (iPSC)
Shinya Yamanaka (2012 Nobel Prize in Physiology or Medicine)
• Yamanaka factors: Oct3/4, Sox2, Klf4 and c-Myc (all transcription factors!)
• Turn differentiated into undifferentiated cells → pluripotency!
• Reprogramme adult cells (e.g., fibroblast obtained from skins) into stem cells
• iPSCs can turn into many different cells with proper factors
Clinical relevance:
• Disease modelling: in vitro models for animal/human diseases (especially for genetic disorders)
• Screening of drugs for treatment
• Derived from patients → CRISPR to correct mutations → potential cell transplantation (no immune rejections
its good cos
iPSCs can be generated from patients with specific diseases and then differentiated into relevant cell types (like heart or nerve cells) to study the disease's molecular mechanisms in a laboratory setting.
this good cos
Since iPSCs can be made from a patient's own cells, they can be used to test how a particular drug will affect that individual's cells, leading to more personalized and effective treatments.
e.g. when myocardial infraction happens there are less stem cells. but what you could do is now inject more cardiomyocytes to replace the lost cells, as they cant regenerate

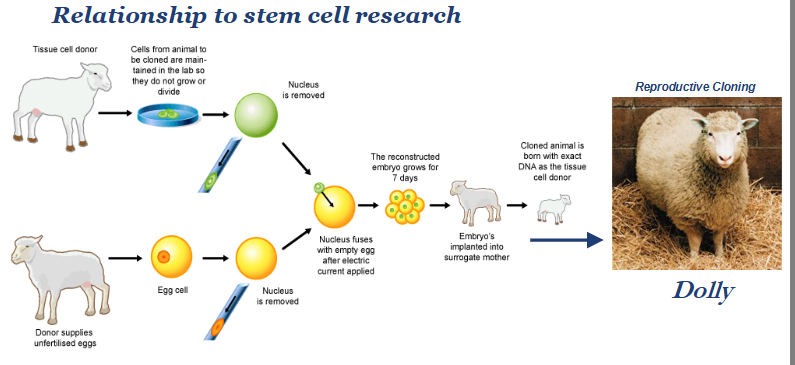
how does cloning work?
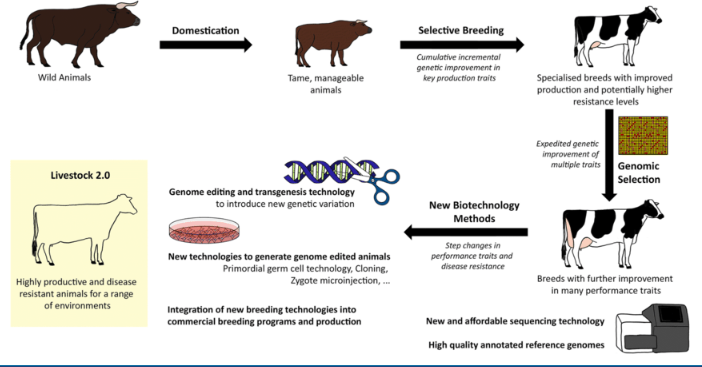
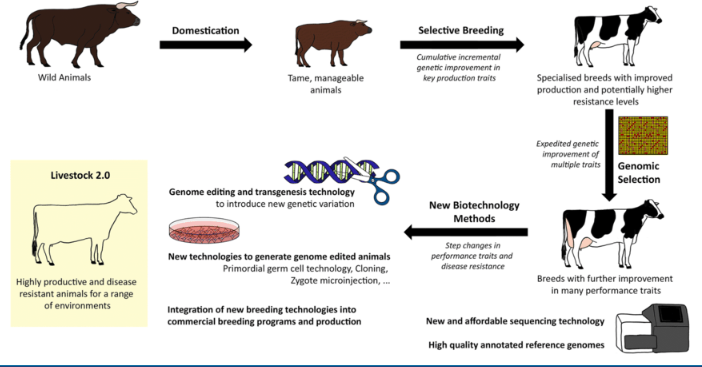
how do we use stem cells in livestock?
use germline stem cells
to create the best breed
• Disease resistance
• Heat/cold tolerance
• Shorter life-cycle
• Better quality of body type
• Market preference
this improves livestock breeding
what are the ethical concerns of stem cells?
Source of stem cells: ethical source?
• Food safety:
Are these “engineered” livestock/food safe to eat?
• Regulatory policy:
Are we well-informed that those are “lab-grown” food or genetically engineered?