6th Grade - States of Matter
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Solid
A state of matter that has a definite shape and a definite volume.
Crystalline solid
A solid that is made up of crystals in which particles are arranged in a regular, repeating pattern.
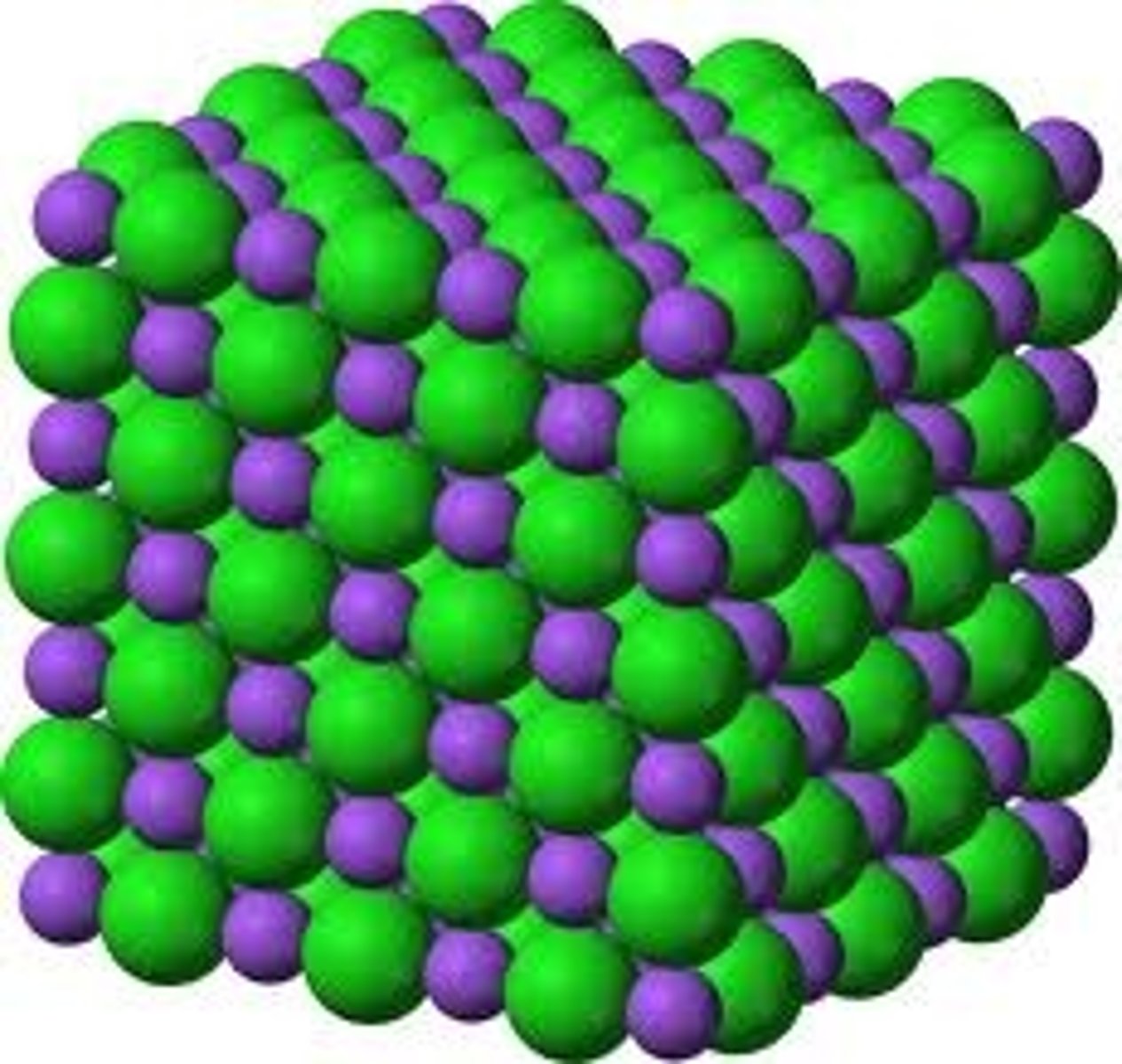
Amorphous solid
A solid made up of particles that are not arranged in a regular pattern.

Liquid
A state of matter that has no definite shape but has a definite volume.
Fluid
Any substance that can flow.
Surface tension
The result of an inward pull among the molecules of a liquid that brings the molecules on the surface closer together; causes the surface to act as if it has a thin skin.
Viscosity
A liquid's resistance to flowing. (ex. honey is more viscous than water)
Gas
A state of matter with no definite shape or volume.
Melting
The change in state from a solid to a liquid.
Melting point
The temperature at which a substance changes from a solid to a liquid; the same as the freezing point, or temperature at which a liquid changes to a solid.
Freezing
The change in state from a liquid to a solid.
Vaporization
The change in state from a liquid to a gas.
Evaporation
The process that occurs when vaporization takes place only on the surface of a liquid.
Boiling
The process that occurs when vaporization takes place inside a liquid as well as on the surface.
Boiling point
The temperature at which a substance changes from a liquid to a gas; the same as the condensation point, or temperature at which a gas changes to a liquid.
Condensation
The change of state from a gas to a liquid.
Sublimation
The change in state from a solid directly to a gas without passing through the liquid state. (ex. dry ice)