Pchem Exam 3 (pKa and chromatography)
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
what is pka
the propensity of a molecule to be protonated; an equilibrium
Equilibrium when pH=pKa
equal amounts of HA and A-
Equilibrium when pH > pKa
solution is more basic so acid loses H more (towards right side); more A- than HA
equilibrium when pH<pKa
solution is more acidic so acid loses H less (towards left side); more HA than A-
pKa of COOH
4.75
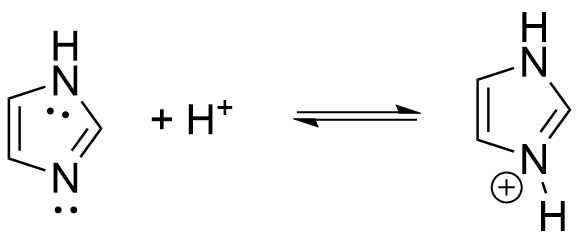
pKa of imidazole
7
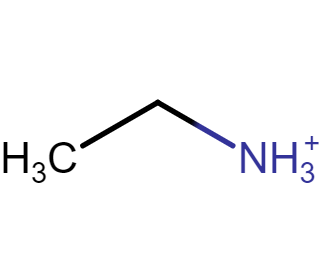
pKa of amine
11
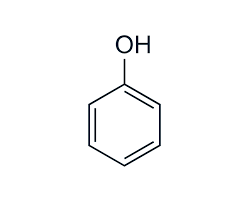
pKa of phenol
10
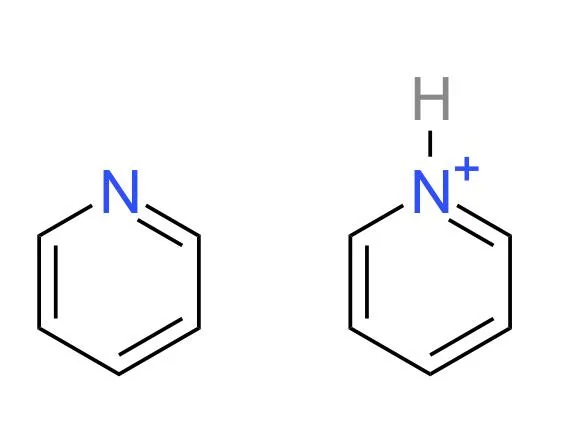
pKa of pyridine
5
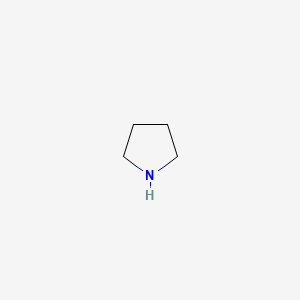
pKa of pyrrolidine
10
if pH > pKa
solution more basic so compound deprotonated
if pH < pKa
solution more acidic so compound protonated
Partition coefficient (K)
g of compound per mL of organic solvent/ g compound per mL water
higher K means what?
more solvent in organic solvent than water
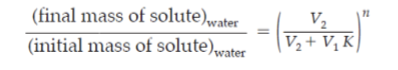
define each variable

neutral species are more soluble in which layer
organic
charged species are more soluble in which layer
water (aq)
to extract an acid into water pH needs to be higher or lower than pKa?
higher (bc then solution wld act as base and steal the H from the acid making it negative so it wld be charged and go into water)
to extract a base into water pH needs to be higher or lower than pKa?
lower (bc then solution wld act as acid and donate H to the base making it positive so it wld be charged and go into water)
pKa of HCl and when wld u use it for seperations
-2; wld use to extract a base
pKa of H2CO3 and when wld u use it for separations
6.5; wld use to extract a stronger acid if you 2 acids want the weaker acid to stay in organic phase to j get the stronger one in water
pKa of NaOH and when wld u use it for separations
14; wld use to extract a weaker acid / acid in general
Chromatography; separation of a molecule is based on chemical structure so what properties?
polarity, functional grp, size, boiling point
4 types of chromatography we go over
thin liquid chromatography (tlc), liquid chromatography, gas chromatography, high performance liquid chromatography (hplc)
TLC stationary phase?
SiO2
SiO2 polarity?
polar
TLC mobile phases?
ethyl acetate (polar) and hexanes (nonpolar) mixture
TLC which wld elute first
nonpolar
TLC which wld have highest retention time
polar
if you increase mobile phase % polar solvent what happens?
lessen interactions with polar stationary phase so move FURTHER/FASTER and LESS separation between molecules
if you increase mobile phase % nonpolar solvent what happens?
molecules interact more with polar stationary phase, move LESS but BETTER separation between molecules
TLC parameters we can manipulate (7)
stationary phase composition
mobile phase composition
flow rate of mobile phase
size of stationary particle
level of packing of stationary phase
amount of stationary particle
height and width
how to get a sharper peak (don’t want broad bc can overlap and can’t tell proper diff between molecules and can’t find AUC)
smaller particles + good packing + optimized flow rate = sharper peaks
calculation of column efficiency
how good is the separation? good is sharp/narrow peaks
theoretical plates (N)
a plate is one “mixing and separating” cycle
if N is large what type of separation
better separation
broader peaks (band broadening) why bad?
the broader the peaks the less efficient the column
sources of band broadening?
Longitudinal diffusion, eddy diffusion, poor set up
Longitudinal diffusion
as it spends more time on column (plate) bands get wider (think the mobile phase pulling compound forward and stationary phase pulling compound the stay in place so getting more stretched with time)
ways to reduce longitudinal diffusion (4)
faster flow rates (less time on plate so less time to spread)
shorter column (less time on plate)
smaller particles (less time on plate bc move faster)
wider column (push mobile phase faster)
eddy diffusion
particles in the column (plate) not all perfectly uniform so different paths exist so molecules take different routes —> peaks widen (since same molecules in compound will elute at diff times)
what resolution is desirable?
> 1.5; want peaks to be as narrow as possible so we can see separation so want 2 signals that meet the baseline
factors that affect resolution? (4)
column (plate) length
stationary phase
mobile phase in LC
temperature in GC
Asymmetry factor formula
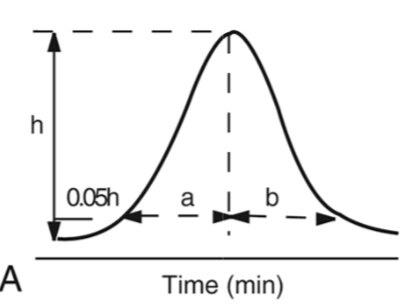
AF = b/a (right side area/ left side area)
AF ideal range
0.95-1.15
fronted asymmetry
A peak that rises slowly and then drops sharply, unlike a symmetrical peak.
causes for fronted
overloading (too much), poor trapping (lack of interactions with stationary phase), injection solvent too strong (HPLC); so think the mobile phase like a wave and washing over and taking everything in the beginning instead of throughout whole time
tailing asymmetry
The peak's front is steep and sharp, while the back gradually slopes down towards the baseline; trailing (back) half appearing broader than its leading (front) half
causes for tailing
adsorption to column stationary phase (sticking to stationary), too much dead volume (mobile phase isn’t sweeping it), common for polar molecules
ion exchange steps
1) deprot (ex the cooh to make neg) or prot (rnh2 —> rnh3+ if not alr positive)
2) bind ionically
3) any other molecules come out
4) change solvent/H2O in excess to wash way the one your trying to purify
Sample Loading: The sample (e.g., hard water or a protein solution) flows through the column, and target ions (positive or negative) bind to the oppositely charged sites on the resin, displacing the original counter-ions (like Na⁺ or OH⁻).
Washing: A buffer (often the same as the loading buffer) is passed through to wash away any non-target molecules or impurities that aren't strongly bound.
Elution: The bound target molecules are released by changing the buffer's salt concentration (e.g., adding NaCl) or pH, which disrupts the electrostatic attraction.
reason for ion exchange
to purify compounds (bc only the charged stuff you want can bind to the stationary phase (ion exchanger) so anything else in the compound will wash away and then u can release j the bound stuff you wanted)
what is cation exchange good for
purifying amines
what is the stationary phase in ion exchange (anion exchange)
mobile anions held near cations that are covalently attached to stationary phase
anion exchange resin, what can bind?
only anions can be attracted to it (resin has + ends)
size(molecular) exclusion chromatography - what elutes first and why?
small molecules penetrate pores of particles where large molecules are excluded (can’t go thru pores) so go around
large will elute first, and smaller take longer to elute
affinity chromatography
one kind of molecule in complex mixture becomes attached to molecule that is covalently bound to stationary phase; all other molecules wash through
what can affinity chromatography be used for
way to purify proteins
how does affinity chromatography purify proteins (what molecules bind to each other)
a protein that has histidine (his6)
the resin you use in stationary phase has nickel covalently bound
1) his6 binds to Ni to all protein you want bound to resin
2) then you wash away all other particles (ex a buffer)
3) then you elute the protein out to get the purified protein (ex use excess histidine/ elution buffer)
HPLC
shorter columns, higher pressure system —> fast flow rate; good to avoid band broadening
HPLC parameters you can manipulate (7)
stationary phase composition (C18)
mobile phase composition
flow rate of mobile phase
size of stationary particle
level of packing of stationary phase
amount of stationary particle
height and width
HPLC parameter limits
pressure and time
HPLC stationary phases
C18 (mostly this), C8, SiO2
how does C18 have compounds stick to it as a stationary phase
hydrophobic interactions, hydrogen bonds, ionic bonds
how does particle size change column efficiency
Efficiency increases with decreasing particle size of the stationary phase
lower particle size —> lower column height —> greater theoretical plates
what does a smaller particle size mean for pressure/ peak
greater pressure/thinner peak
particles that elute at a later will hav what type of peak
broader, and the ones that elute first thinner peak
reverse chromatography stationary and mobile phases
sp: c18
polar mobile: water
nonpolar mobile: methanol, acetonitrile
normal chromatography
sp: sio2
p mp: isopropanol, methanol, ethyl acetate
np mp: hexanes
isocratic and why good and bad
same mp concentration whole time; good for easy separations but can result in longer elution times; able to separate early peaks or late peaks but not both
gradient and y good
concentration varies overtime (you wld slowly add more of the mp that the plate is so if the plate it polar wld slowly add more and more ethyl acetate conc in ur mobile phase)
better separation and can still see compounds that elute later
polarity ranking low to high of normal phase chromatography
hexane, dichloromethane, isopropanol, methanol
polarity ranking low to high of reverse phase chromatography
tetrahydrofuran, acetonitrile, methanol, water
for reverse phase if you increase polar mobile what happens/?
the np molecules will stick more to the np plate so spend more time on column so acc worse off cuz more longitudinal diffusion
hplc relationship between pressure and solvent
the more viscous the solvent the higher the pressure on the system
water—> very viscous (higher p)
MeCN —> less viscous (lower p)
effect of pH
can modulate pH to change separation; pH can affect the separation and resolution of compounds when the compounds pKa is close to that of the eluent (mobile phase); mostly used in reverse phase
effect of temp
retention time (capacity factor) decreases with increased temp, greater peak efficiency with higher temp
increase temp decreases elution time and selectivity/retention
HPLC detection methods
UV, refractive index, evaporative light - scattering, fluorescence, mass spec, fourier transform infrared
AUC is proportional to what
concentration (peak needs to go to baseline to find AUC and therefore conc)
AUC (y axis) vs concentration is called what
calibration curve
peak height whats used for?
nothing it don’t matter
hplc applications: external standard
use the pure compound and make calibration curve to find amount of it in your compound
1) run formulation HPLC: crush pill, take small amount, dissolve in eluent, run on HPLC
2) generate calibration curve by using pure standard at diff concs
3) use calibration curve to determine conc and amount of acc pure standard your looking for in tablet
hplc applications: internal standard how shld u choose it
closely related in structure (same func. grp) and therefore elute close to analyte and result in similar detector response
stable
Chromatographipally resolved from analyte and impurities
hplc applications: internal standard
1) add known amount of internal standard
2) use AUC to compare concentrations (compare ratio of peaks)
DONT need to make a calibration curve both internal standard and compound you investigating on same HPLC
GC mobile phase name and examples
carrier gas: H2 (more common), He2(more common), N2
GC stationary phase
SiO2
how GC works
analyte is injected through a septum into a heated port and evaporates (vaporize)
analyte then moves thru column by carrier gas
GC requirements
molecules must be sufficiently small/volatile (so can evaporate, < 250 g)
column is very long but flow rate is very high
parameters to manipulate in GC
column temp; as you heat molecule interacts less w stationary phase bc moving faster
lower temp for GC means what for resolution
better resolution
column length in GC
generally very long, longer columns allow lower temp
column diameter in GC
smaller the better (small pore so wtv goes thru can interact w stationary phase)
flow rate in GC
high flow rates
GC detectors
flame ionization detecter (most common), mass spec
flame ionization detector
since the molecules are coming off as a gas if you introduce a flame the gas —> ions and detected by ion detector
whats a benefit of the GC detectors
don’t need UV active molecules (FID, MS) and can do with any small molecule