Particle theory: Particles: Chemistry: (9:1)
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
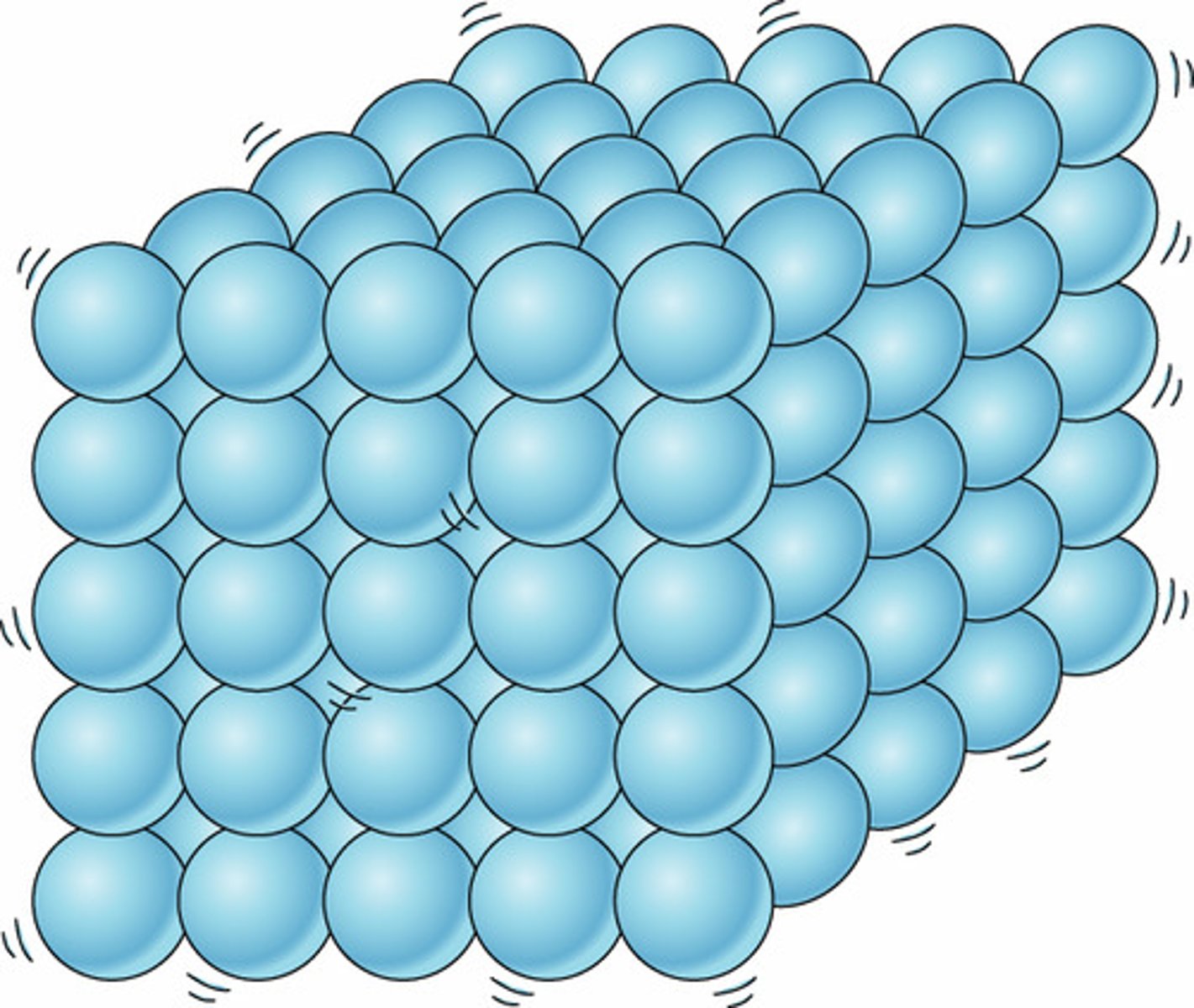
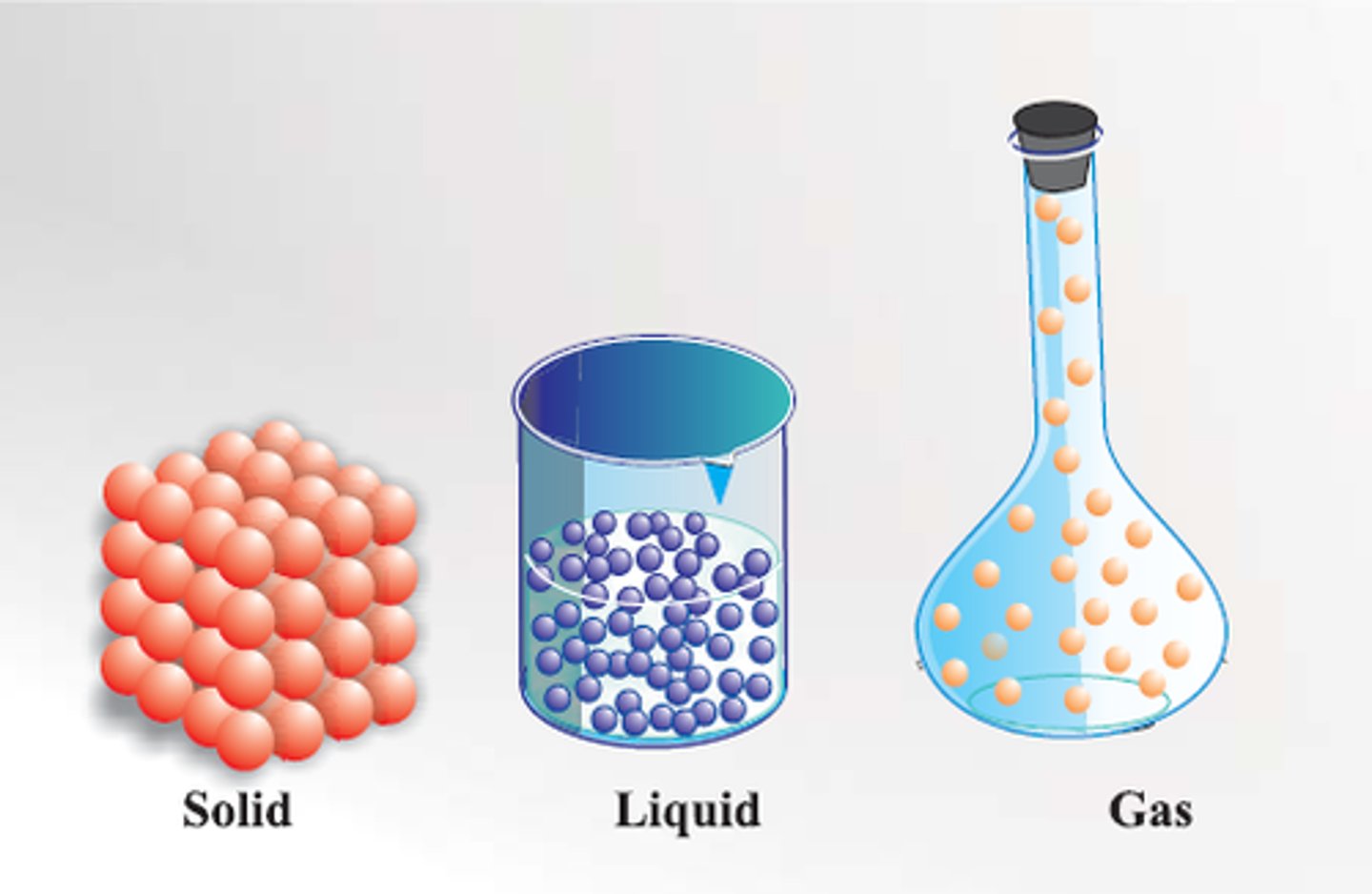
Solid
Definite shape and volume
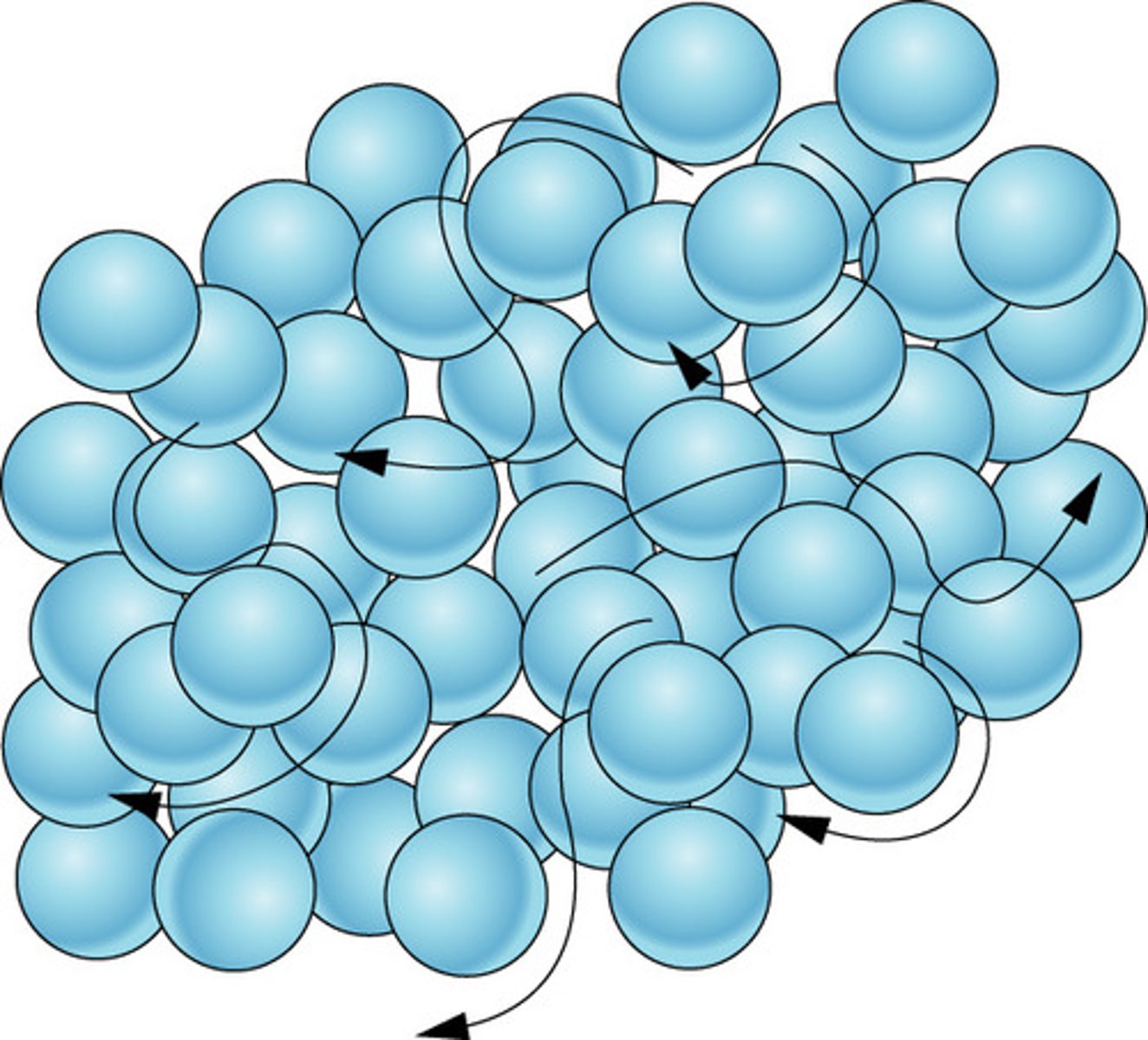
Liquid
Definite volume but no definite shape
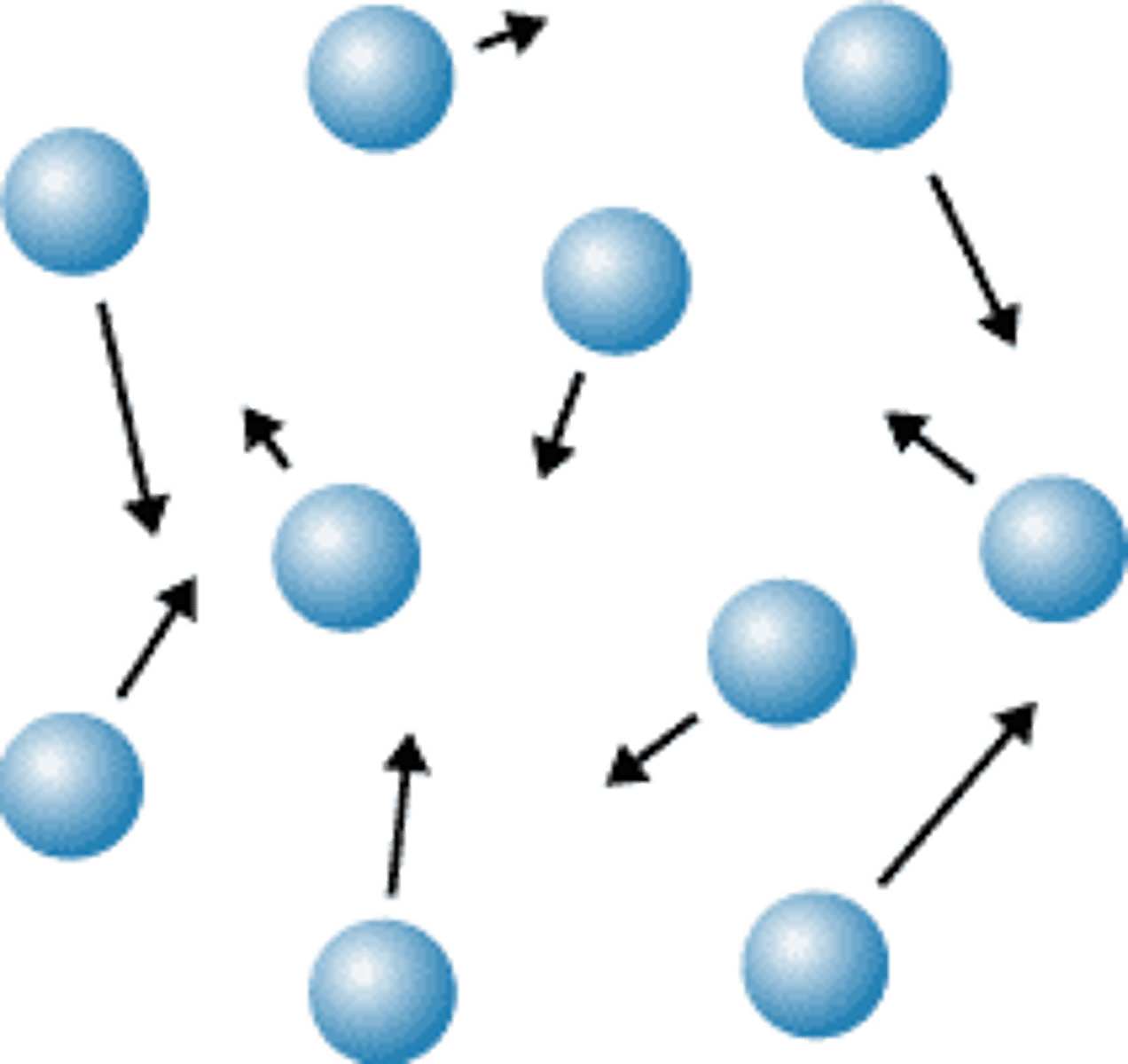
Gas
No definite shape or volume
Melting point
The temperature at which a solid becomes a liquid

Boiling point
The temperature at which a liquid changes to a gas

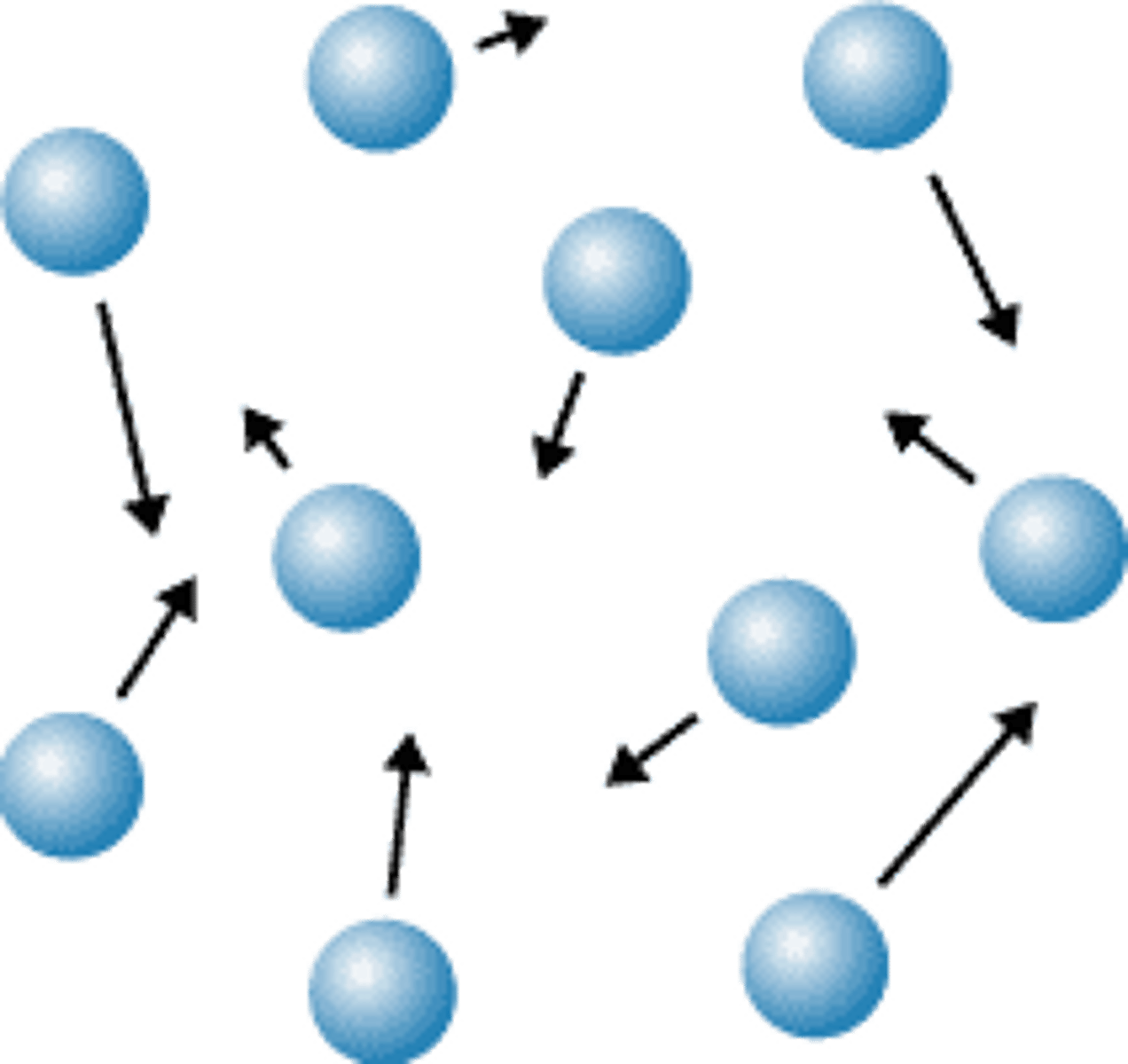
Particle theory
Matter is made up of tiny particles which are represented as small solid spheres which are constantly moving.
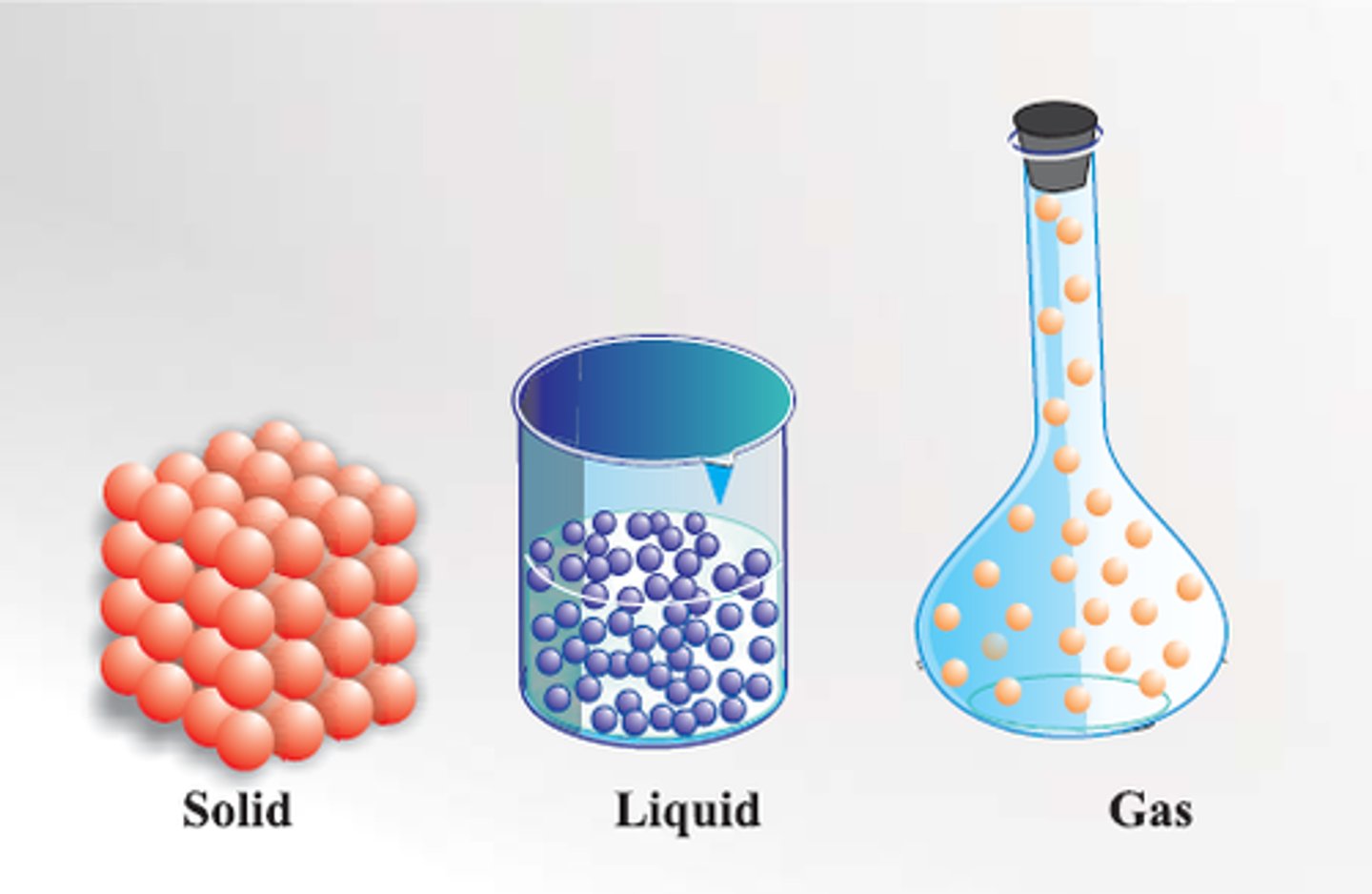
Uses of particle theory
Explaining changes of state
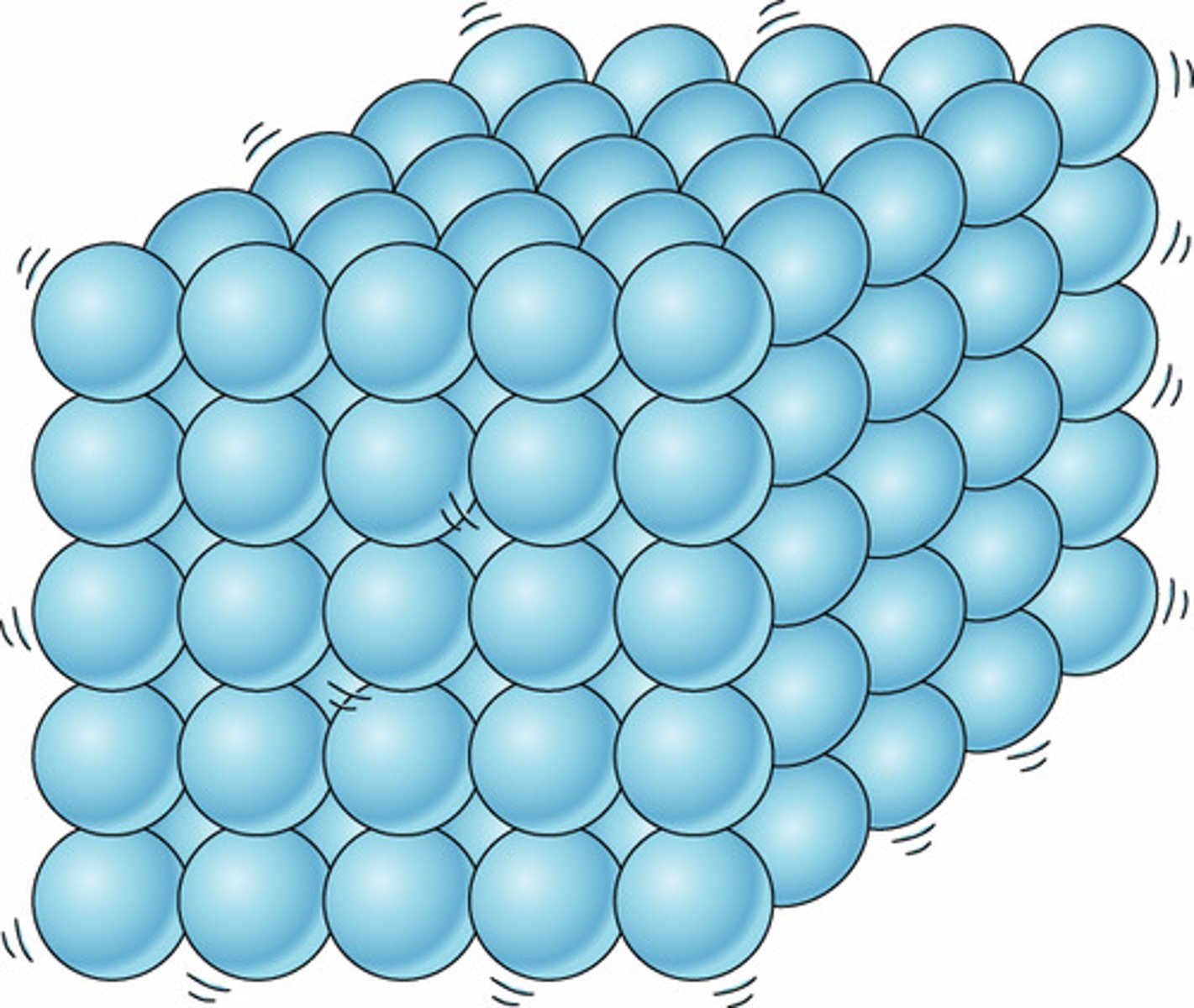
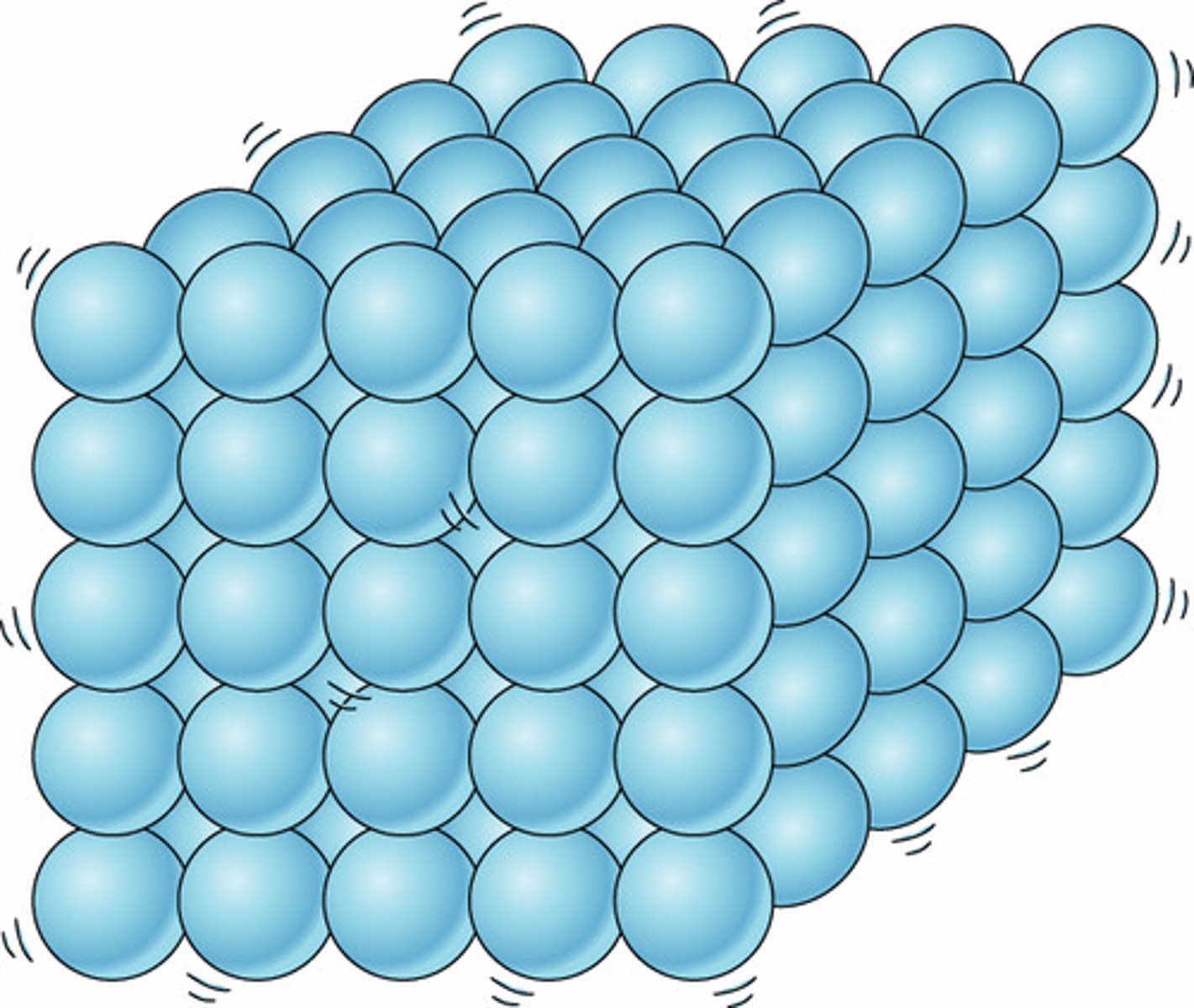
Movement of particles in a solid
Vibrate about a fixed position
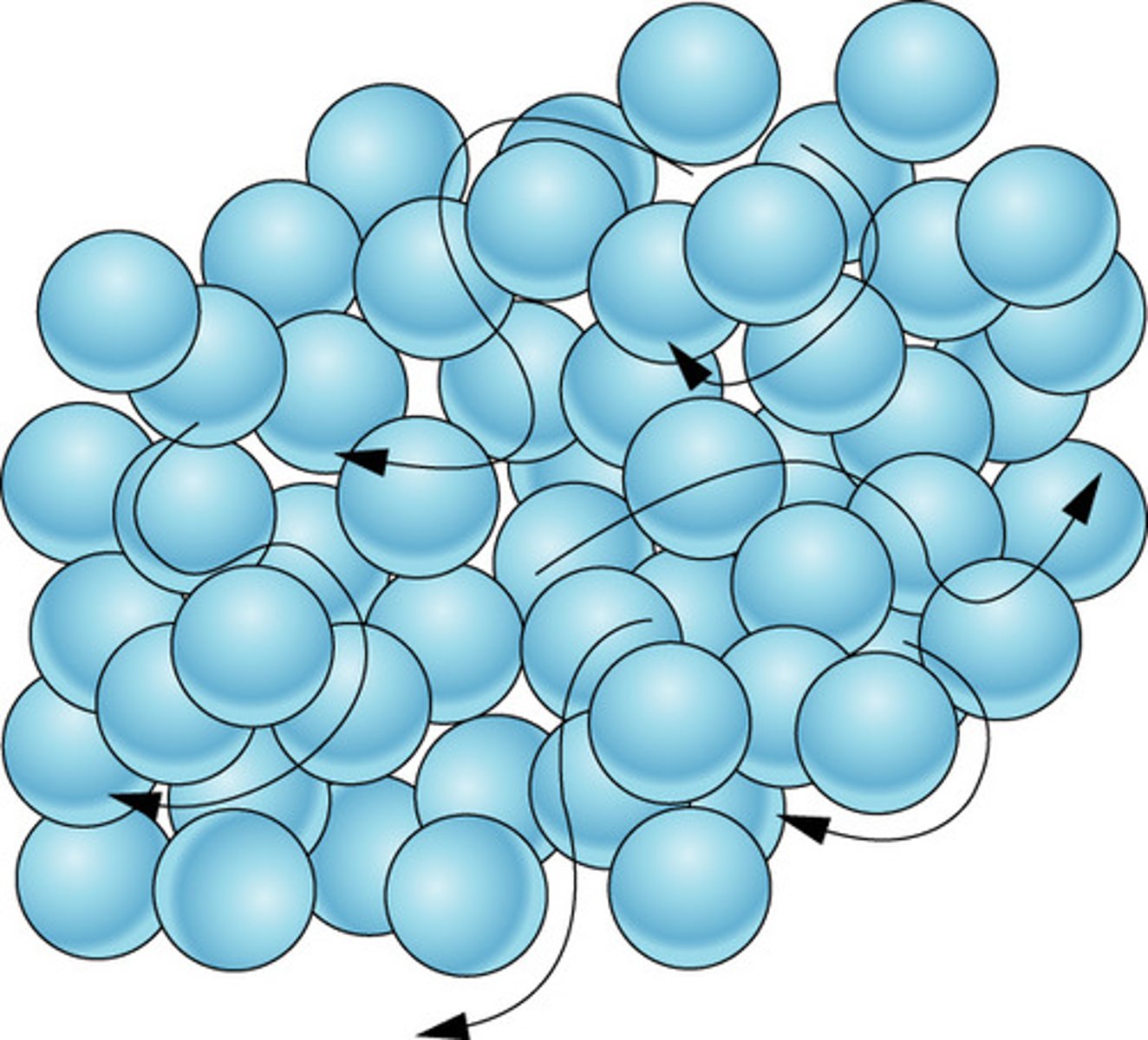
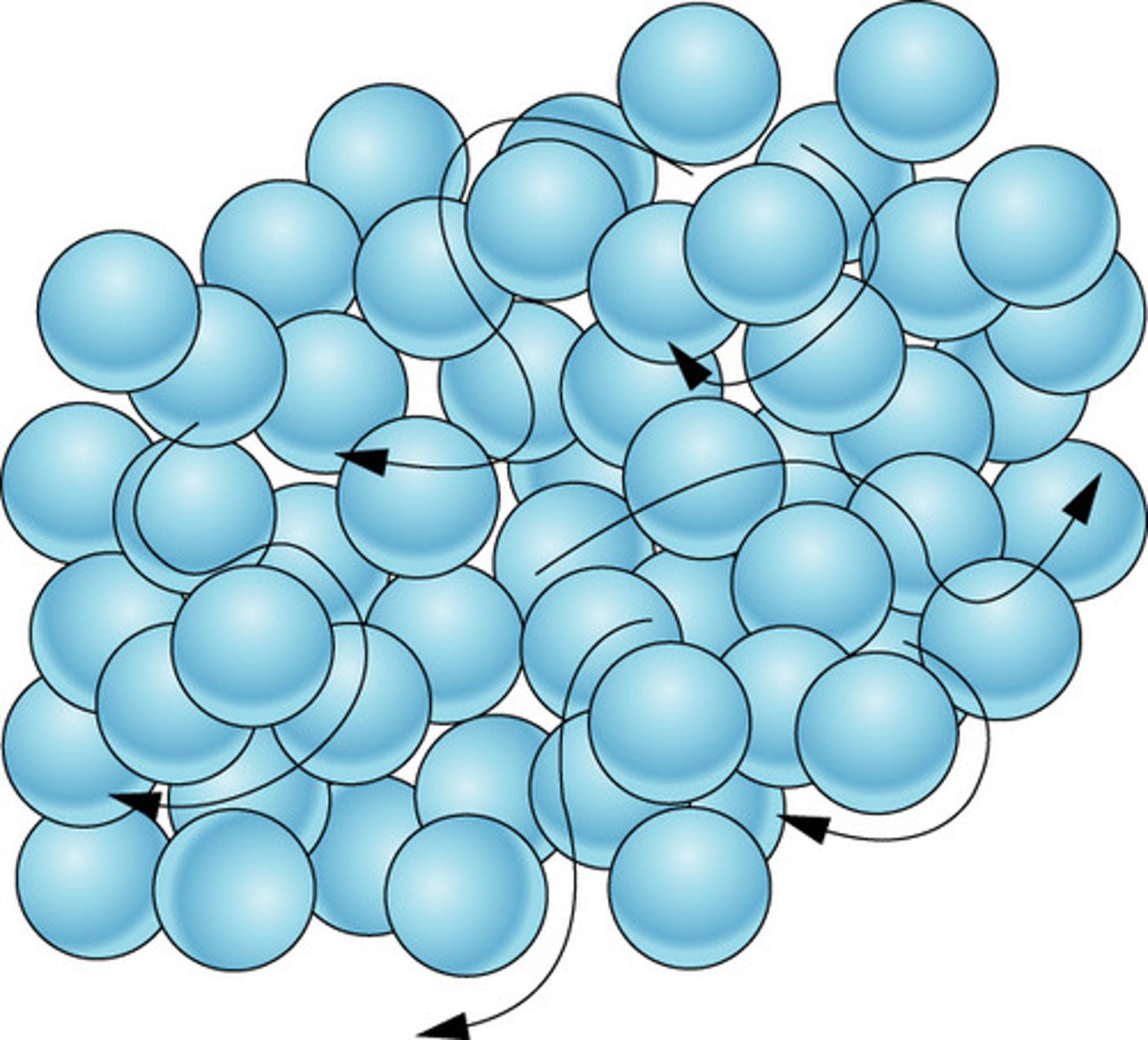
Movement of particles in a liquid
Move around and slide past one another
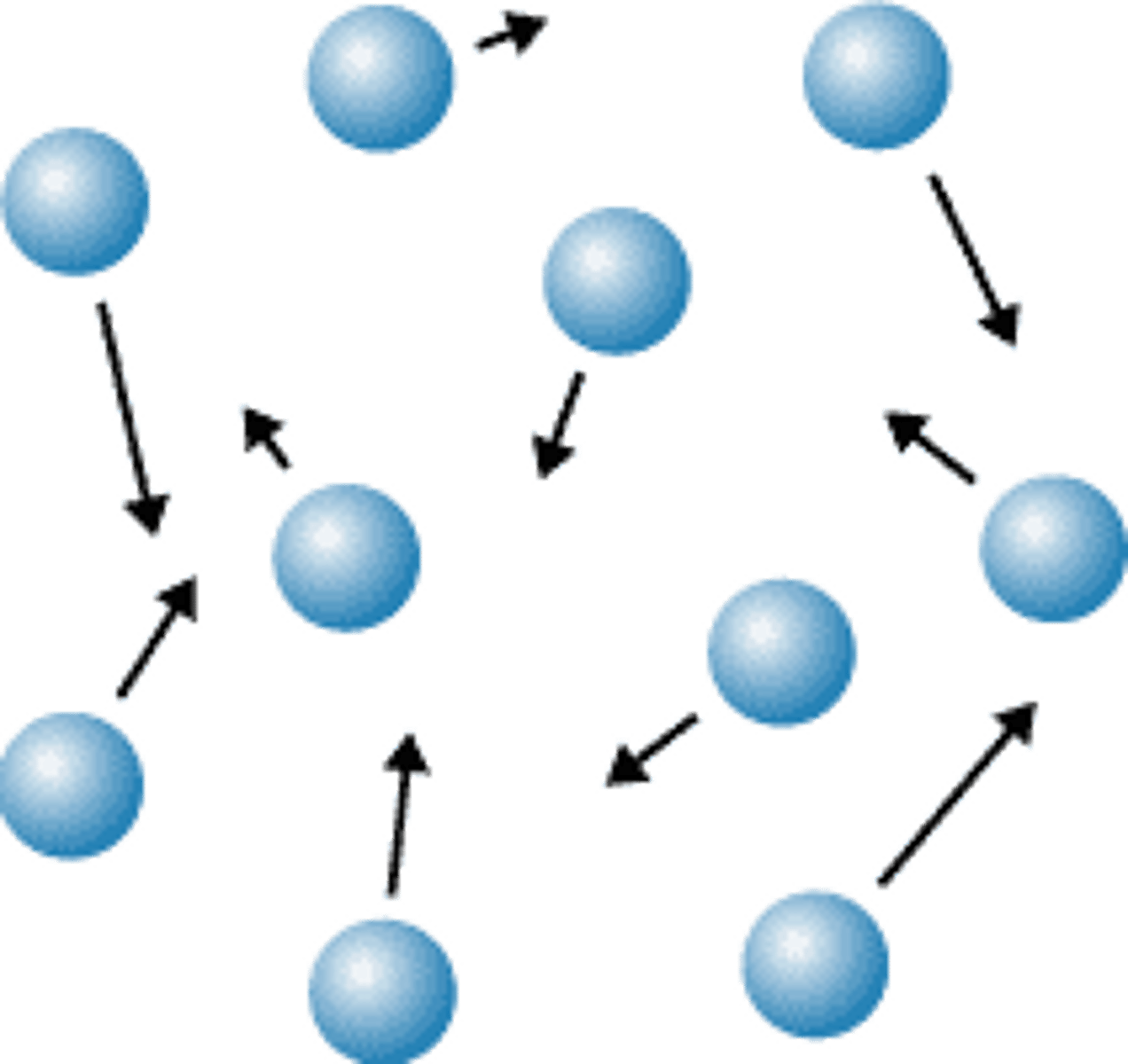
Movement of particles in a gas
Move freely and quickly in all directions
Limitations of particle theory
Does not take into account forces between particles, size of particles, space between particles
Solid --> Liquid
Melting
Liquid --> Solid
Freezing
Liquid --> Gas
Boiling
Gas --> Liquid
Condensing
Matter
Anything that has mass and takes up space
(s)
Solid
(l)
Liquid
(g)
Gas
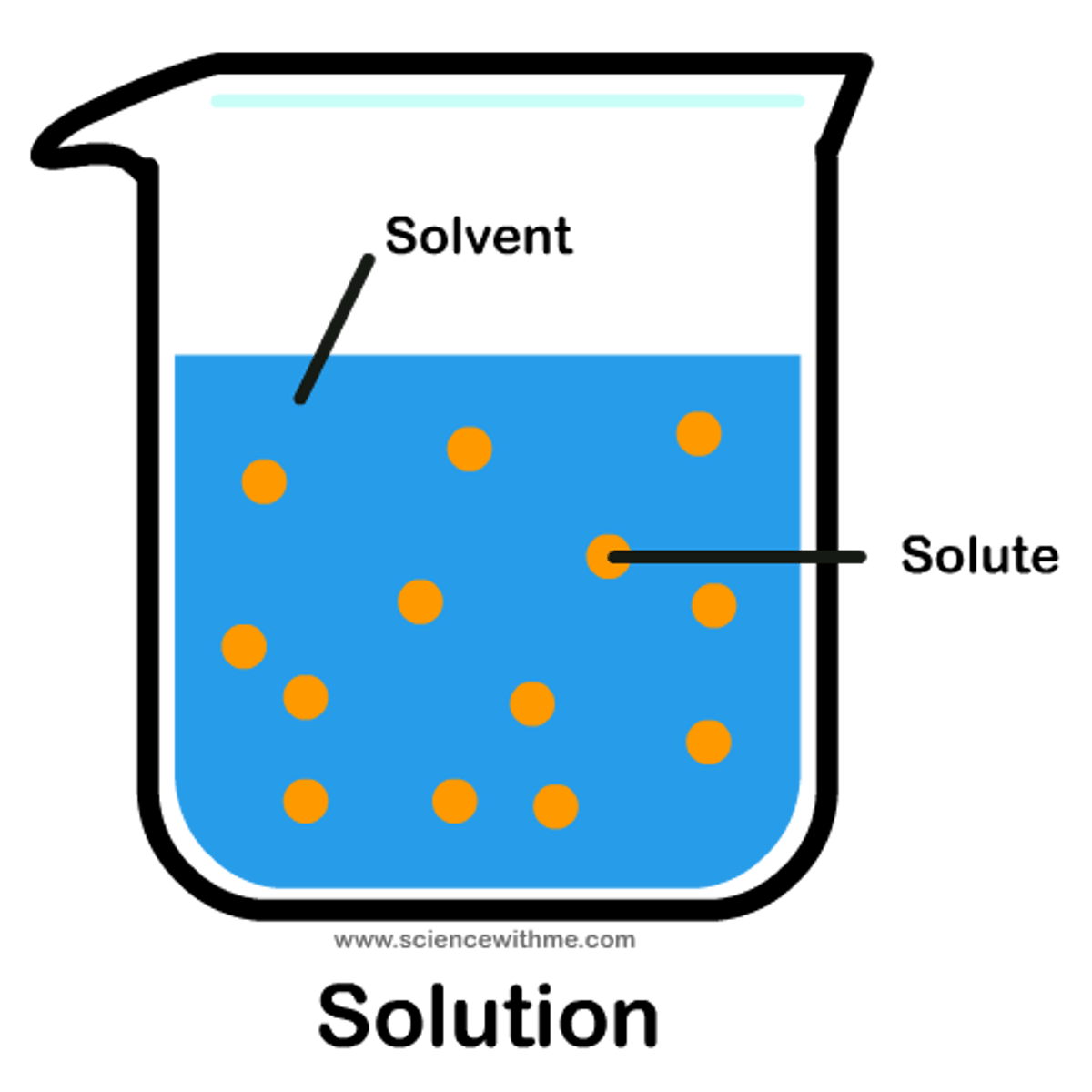
(aq)
aqueous (dissolved in water)
When a substance is heated
Particles stay the same size but move further apart, causing the substance to expand
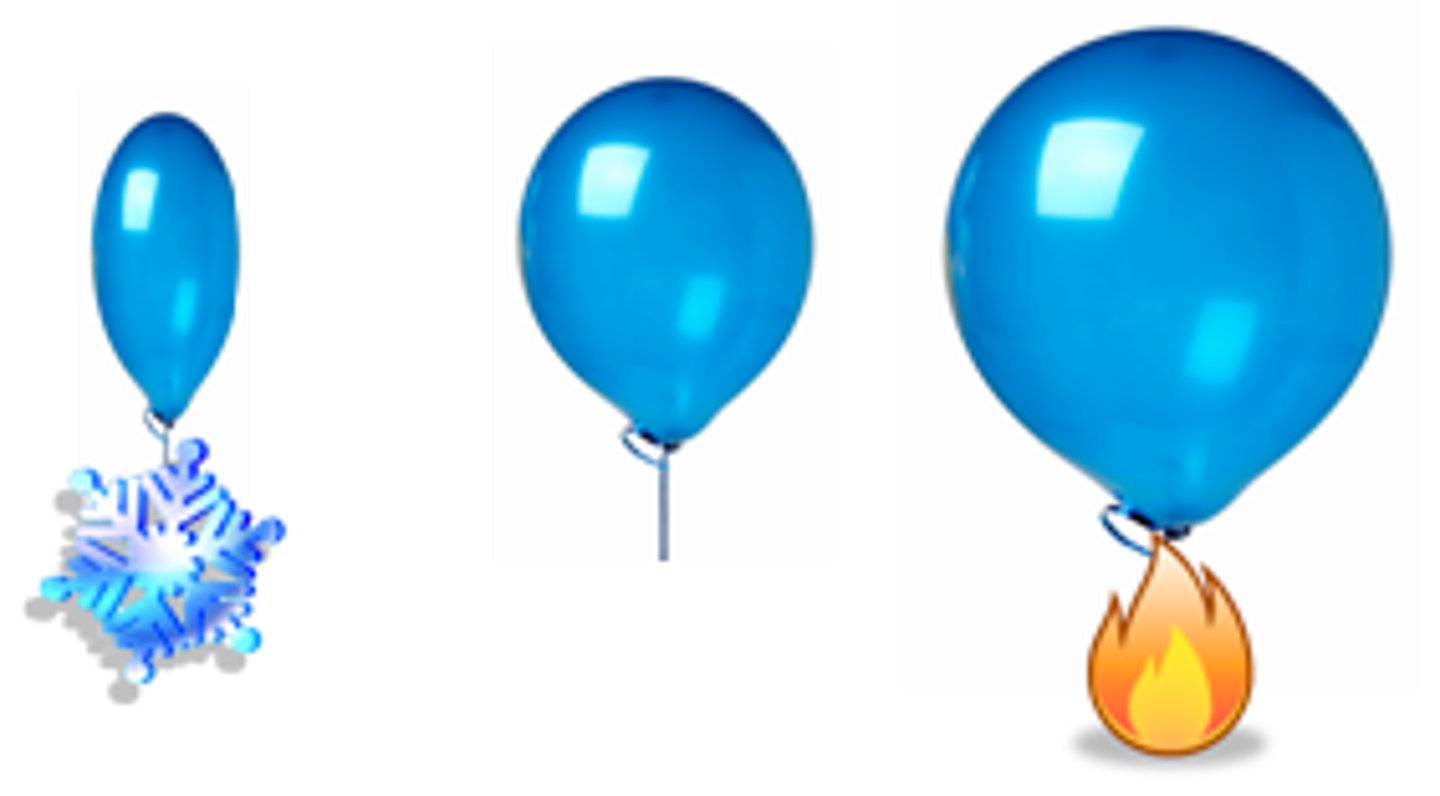
When a substance cools
Particles stay the same size, but move closer together, causing the substance to contract