Chemistry: Equations and Practice (Module 1: Kinetics)
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms


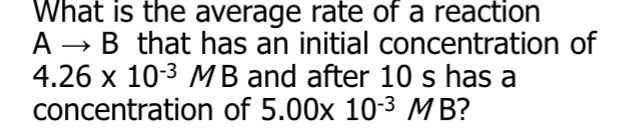
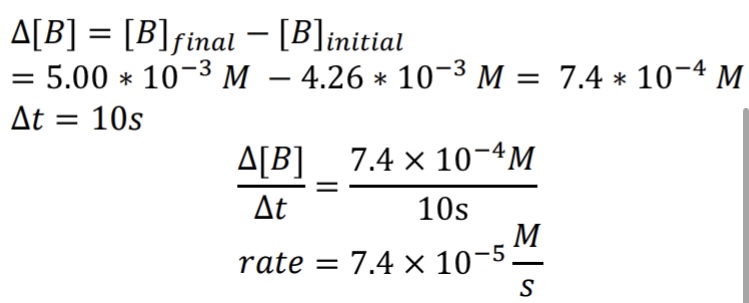

A.

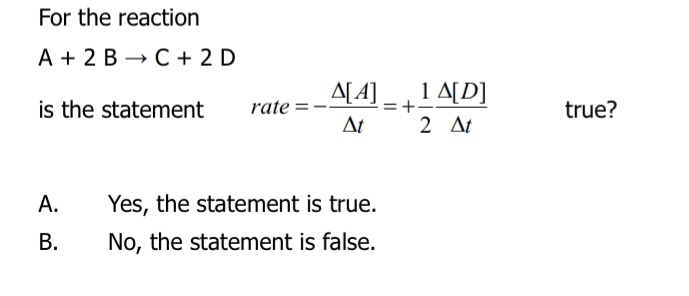
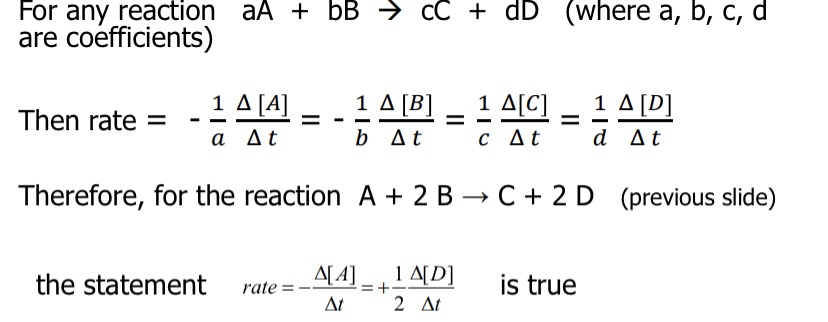
Yes, the statement is true
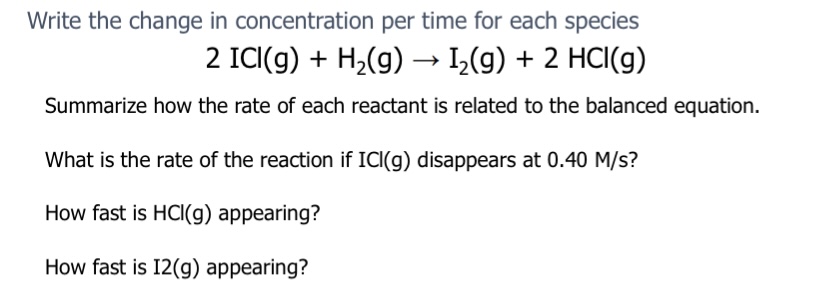
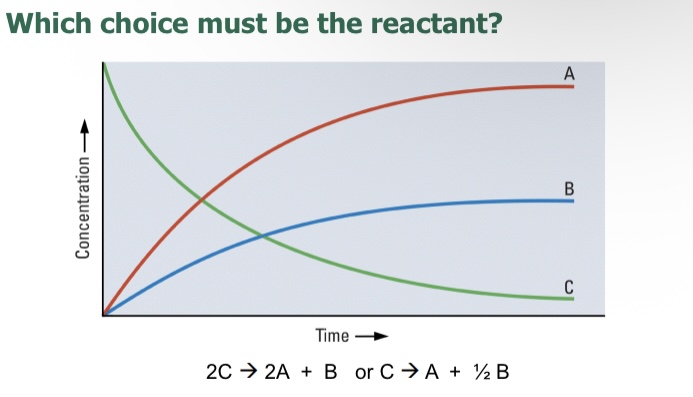
C because it starts as a high concentration but then steadily decreases over time
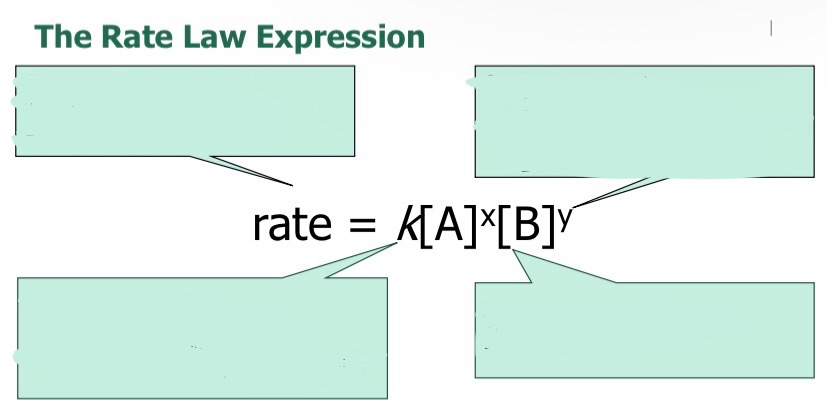
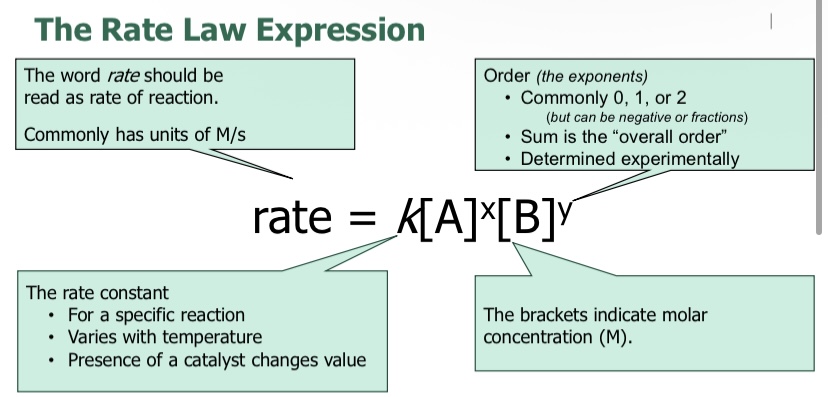
What is the equation when finding the rate law?
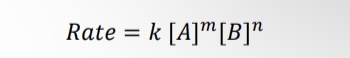
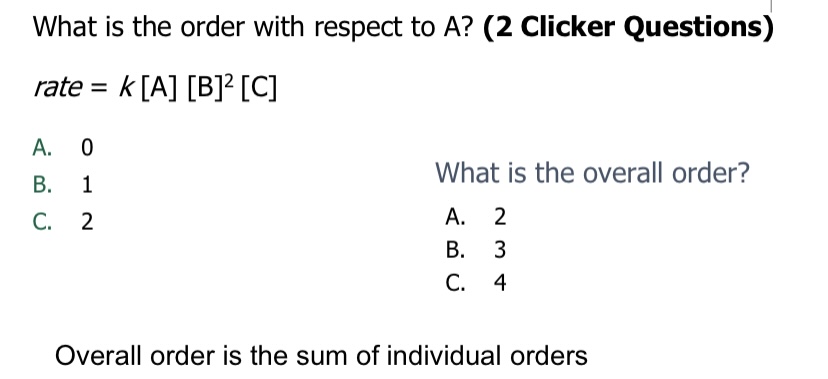
For A, it’s 1
Overall order= 1+2+1=4
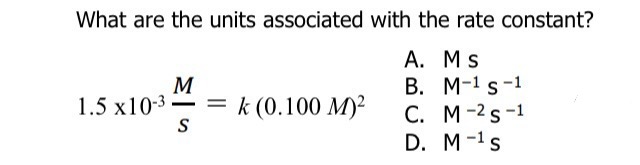
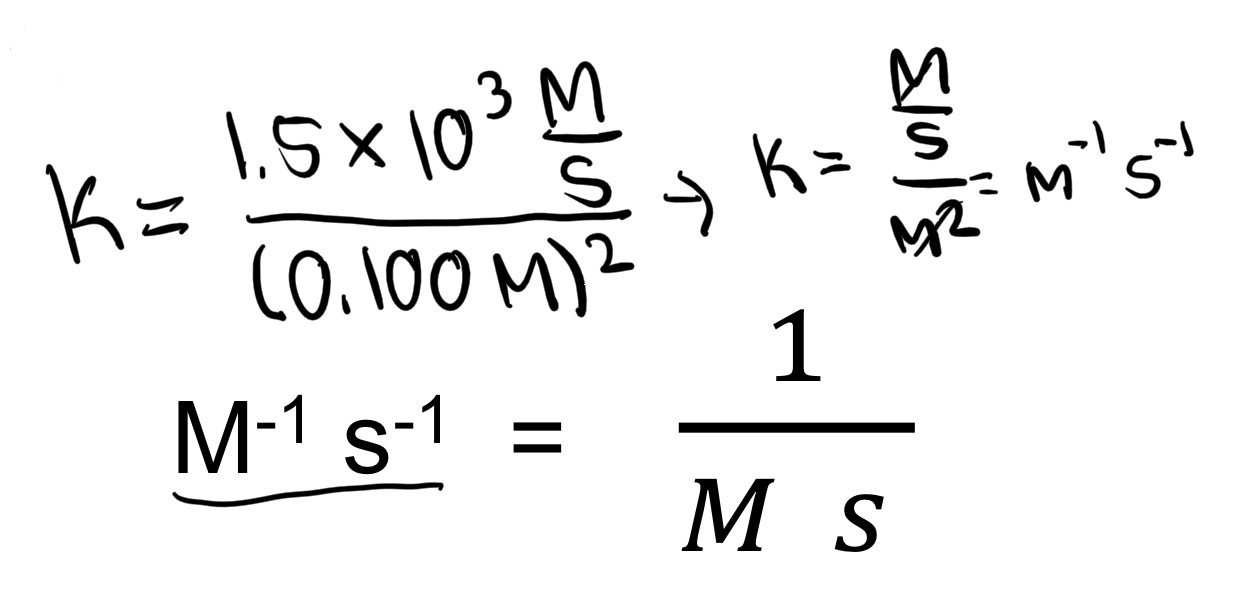

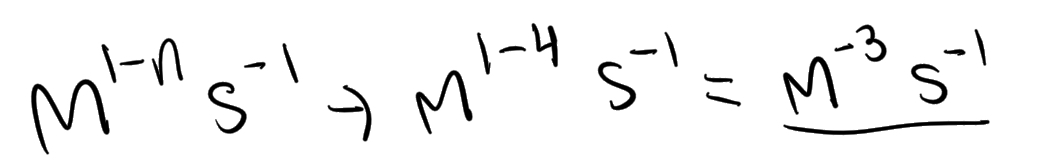
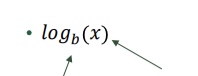

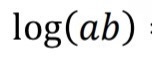
What does this become?
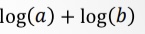
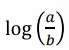
What does this become?
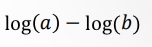
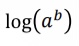
What does this become?
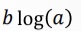
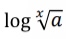
What does this become?
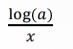

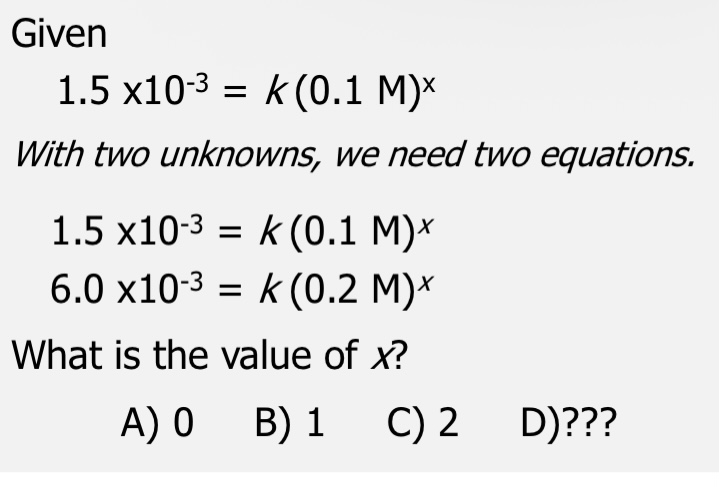
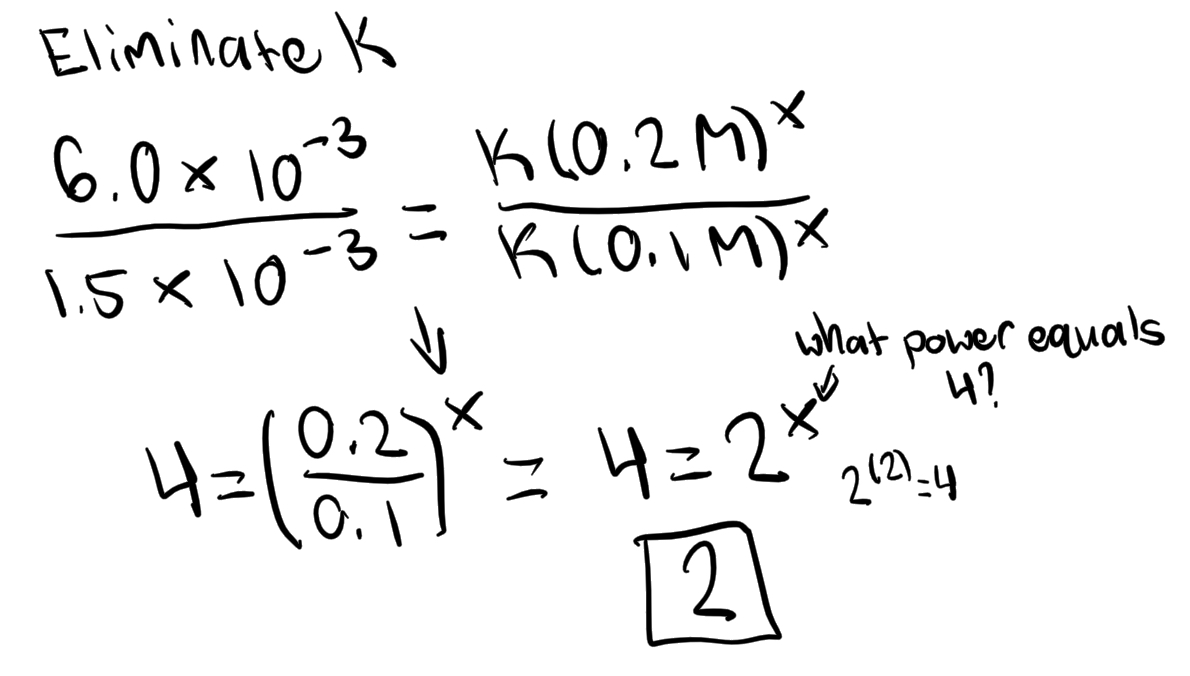
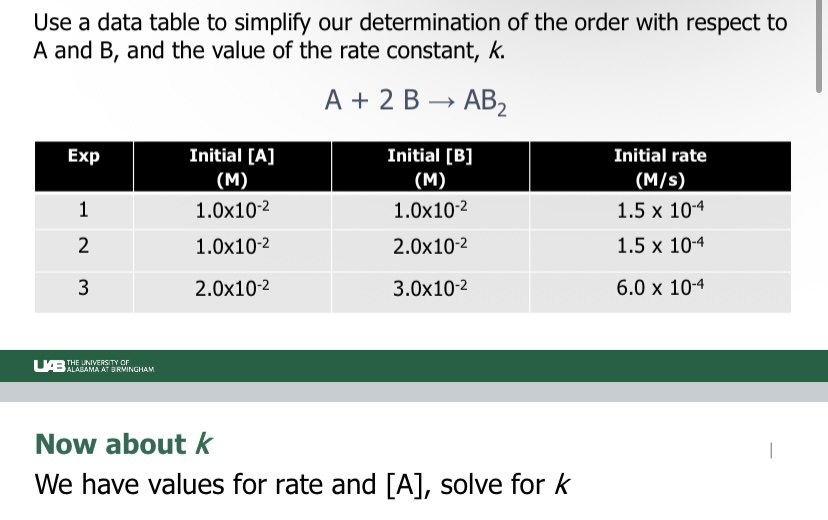
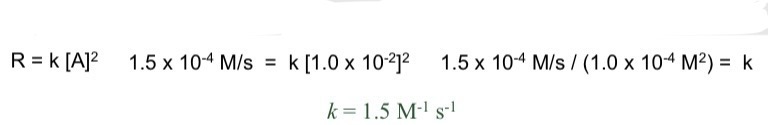

Cannot be determined: Cannot determine simply from the coefficients of the balanced equation
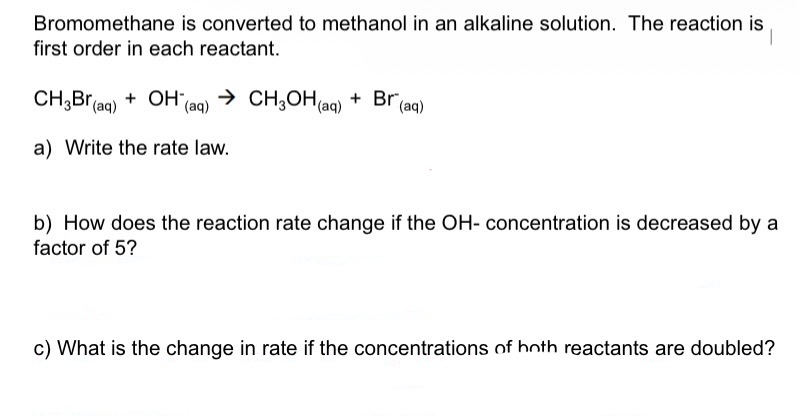
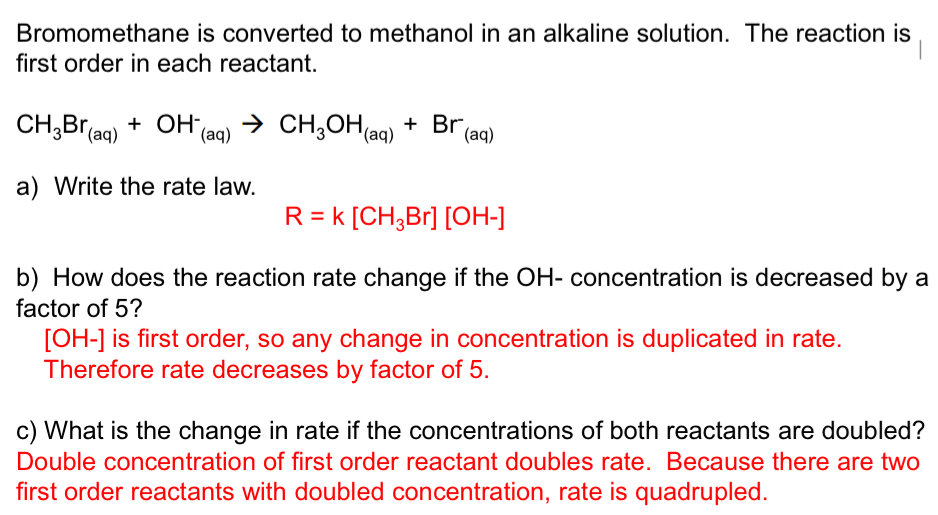
What is the rate law for First Order Reactions?
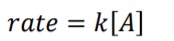
What is the equation for the average rate of reaction?
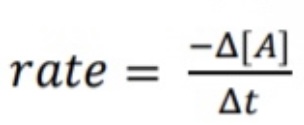
What equation is used to find the rate of change (decreases)?
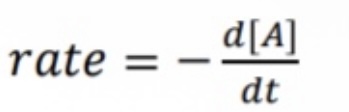
What equation is used for differential rate law for first-order reaction?
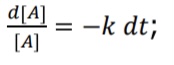
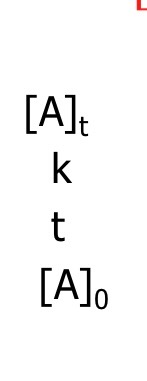

What is the equation (linear form) for 1st order integrated rate law
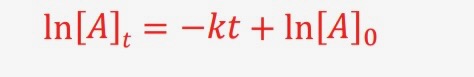
What is the equation (alternate form) for 1st order integrated rate law
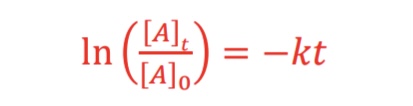
What is the rate law for a second-order reaction?
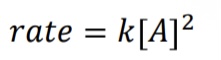
What equation is used to find the integrated rate law for a second-order reaction?
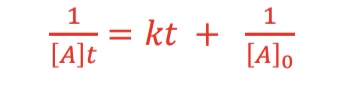
What equation is used for the integrated rate law for a zero-order reaction?
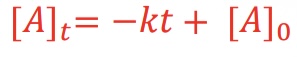
Look over the box



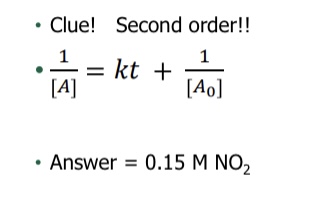
What equation is used for finding half-life for first order reaction?
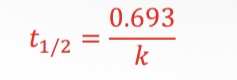
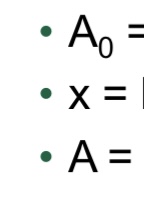


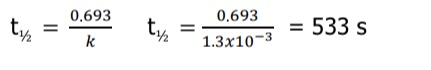
What equation is used for exponential decay related to half-life?
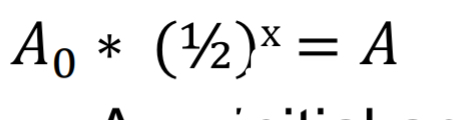

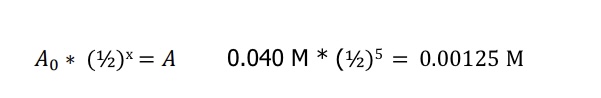
What equation is used to determine the activation energy based on temperature (Arrheinus equation)?
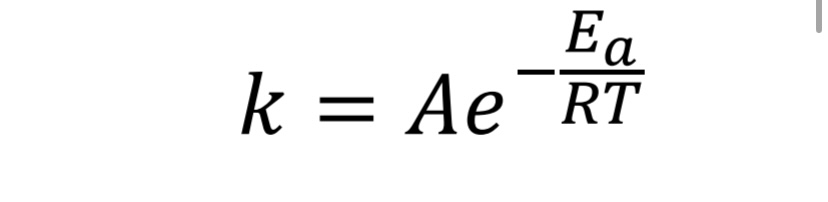


What equation is used to determine the activation energy also and temperature (Arrhenius equation)?
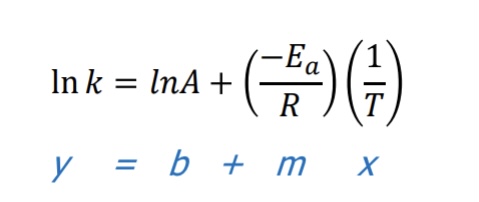
What equation is used when using two different temperatures (Arrhenius equation: Two-point form)
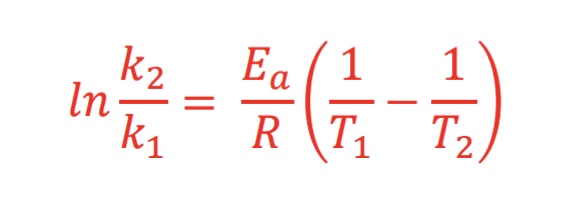
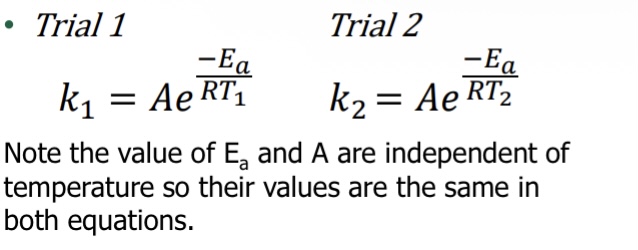
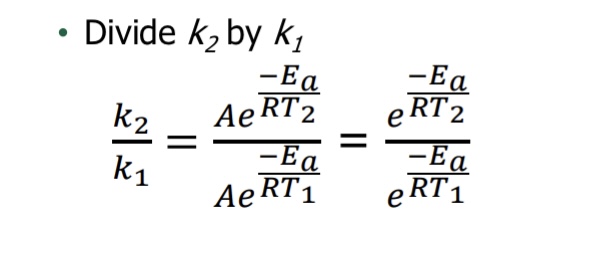

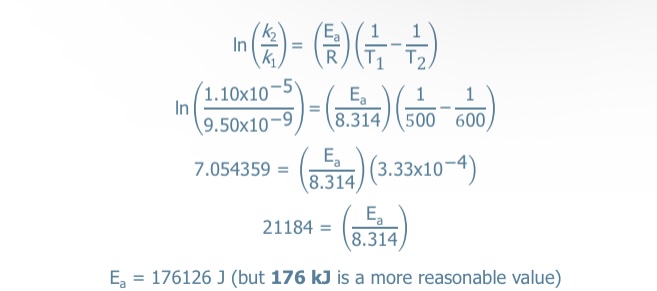

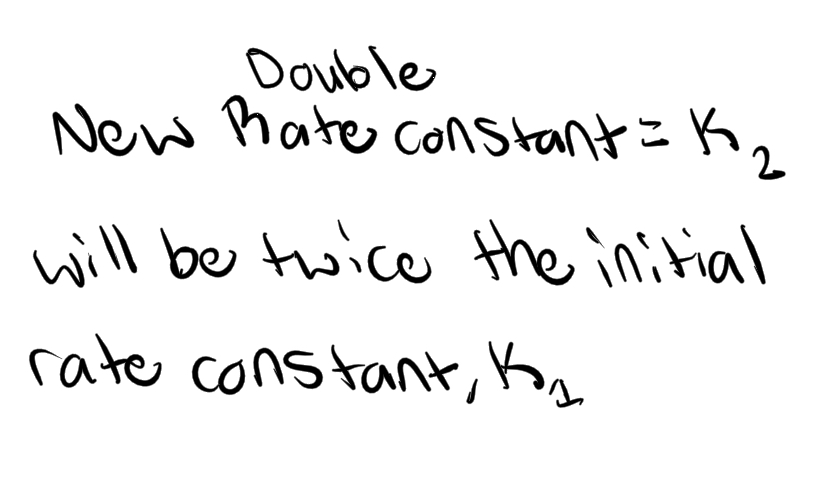

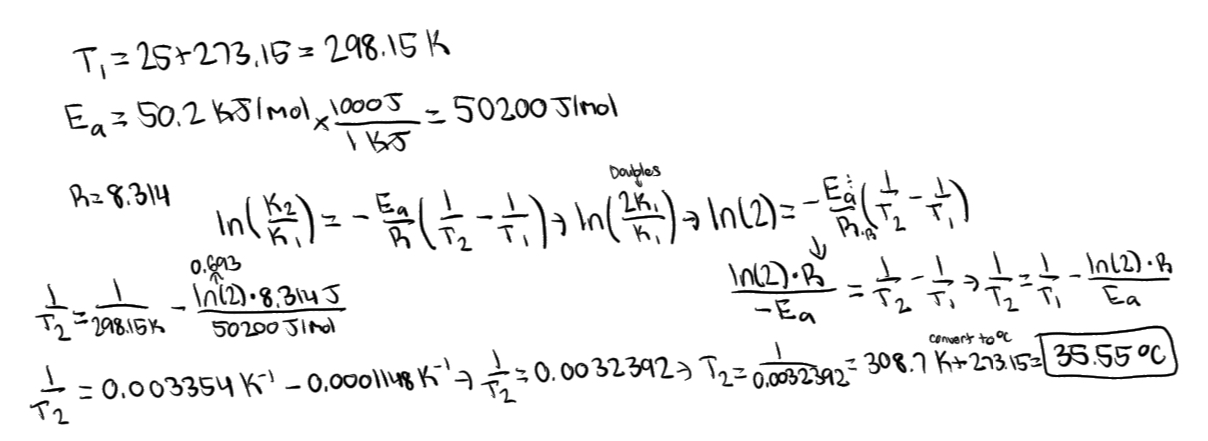
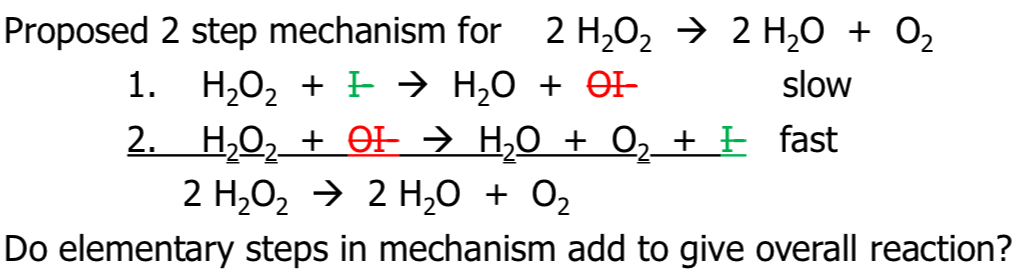
Yes! Necessary requirement for likely mechanism
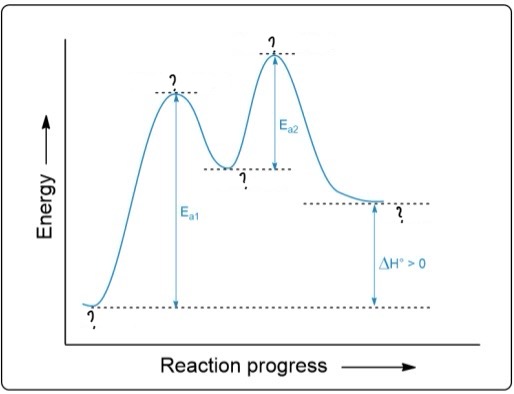
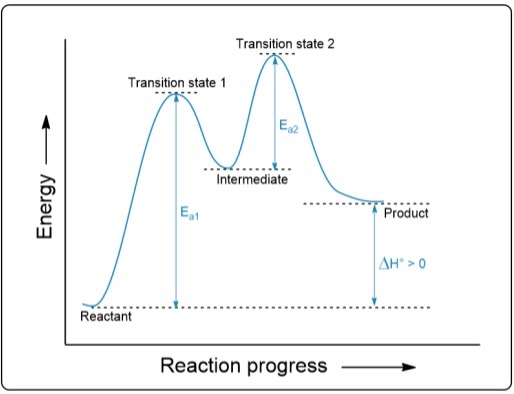

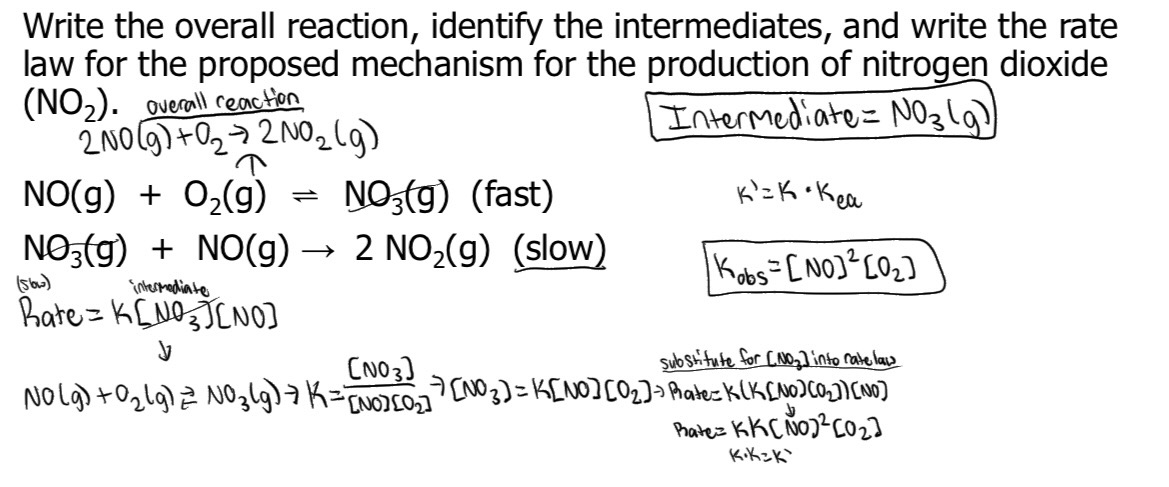
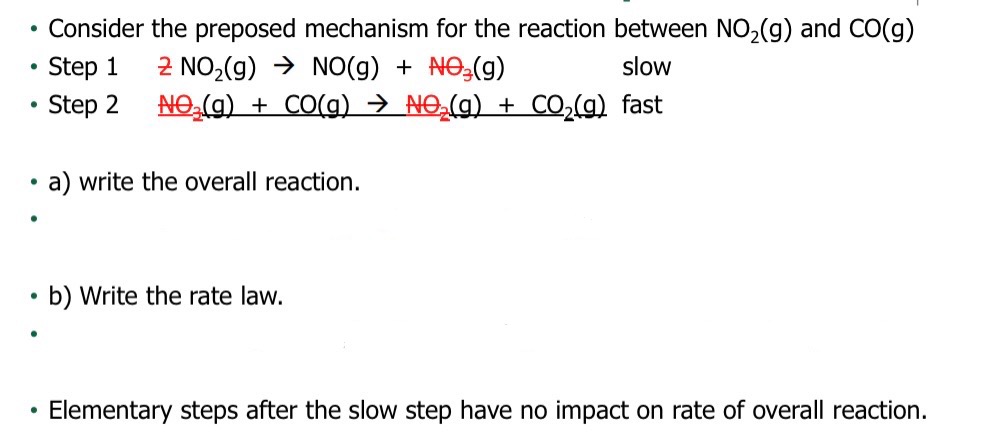
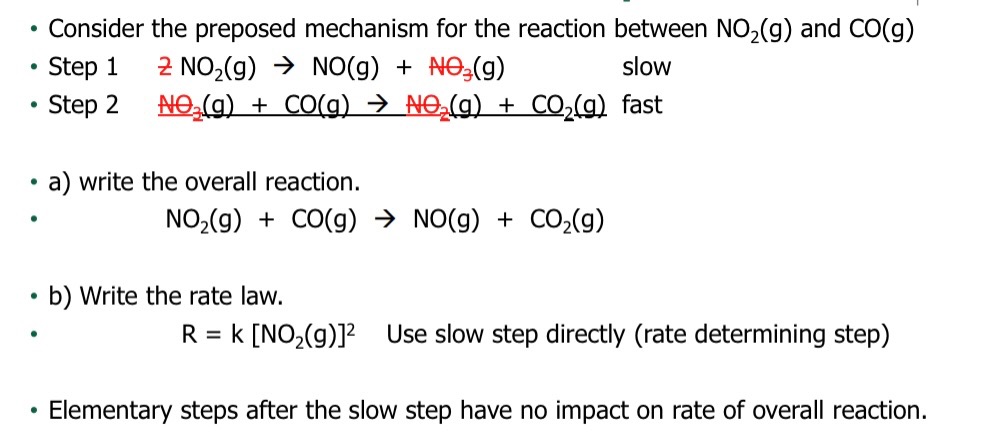
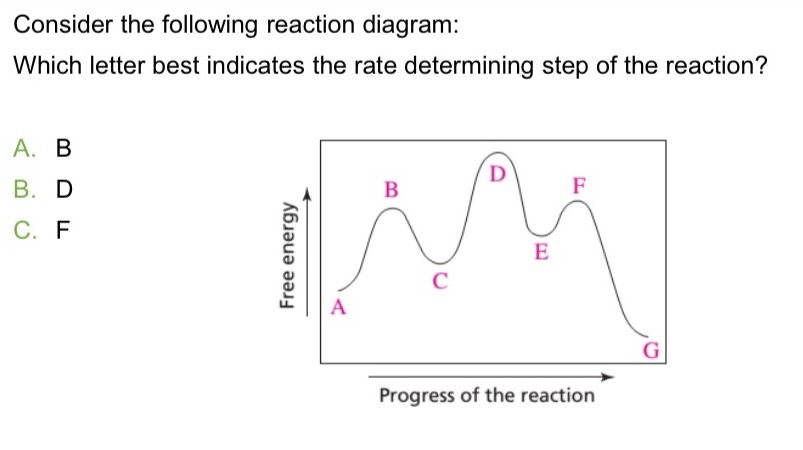
D: Is the highest activation energy in the whole graph
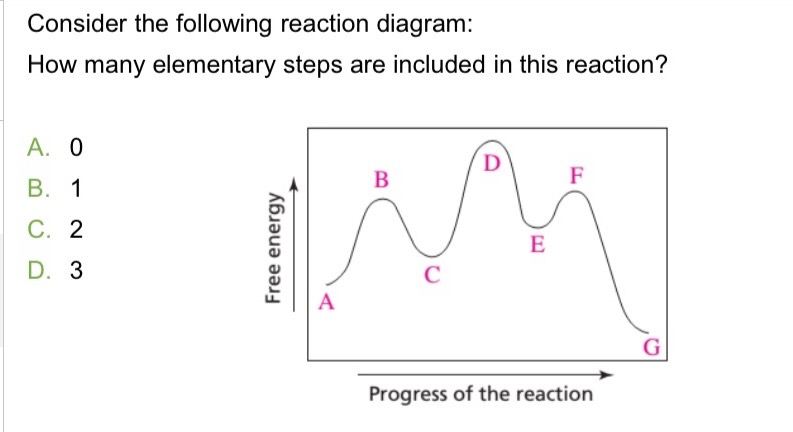
It was B, D, and F (Which means its 3 in total)
You can find the rate law by
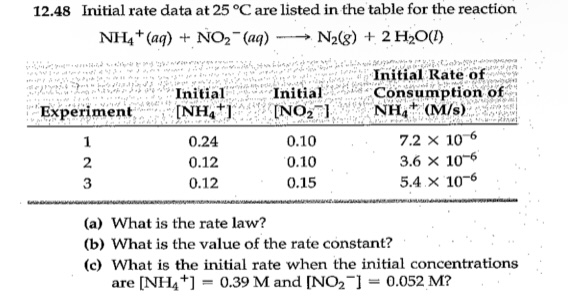
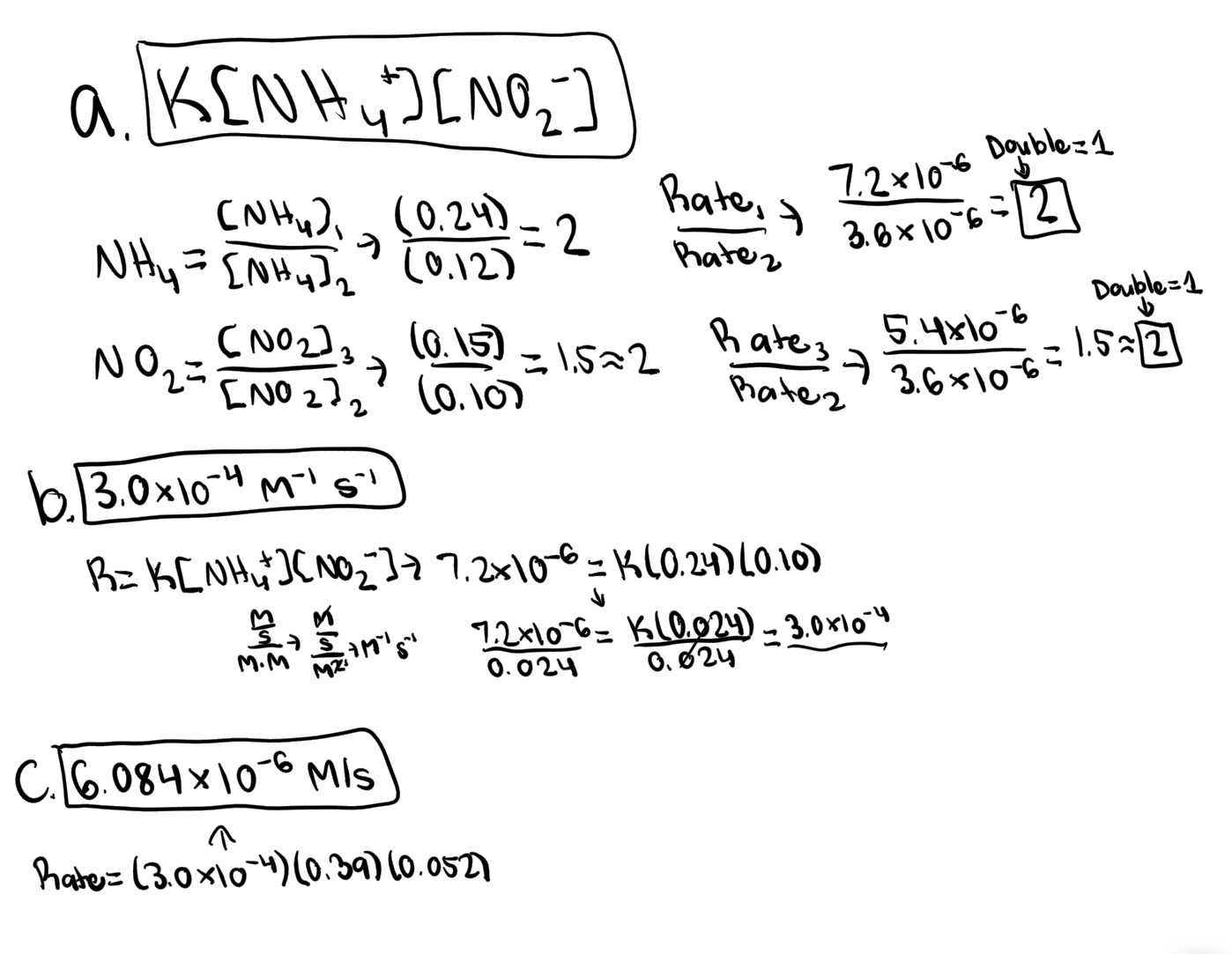
Experiments