StemUp: OCR A A level Physics 6.4: Nuclear and particle physics
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
103 Terms
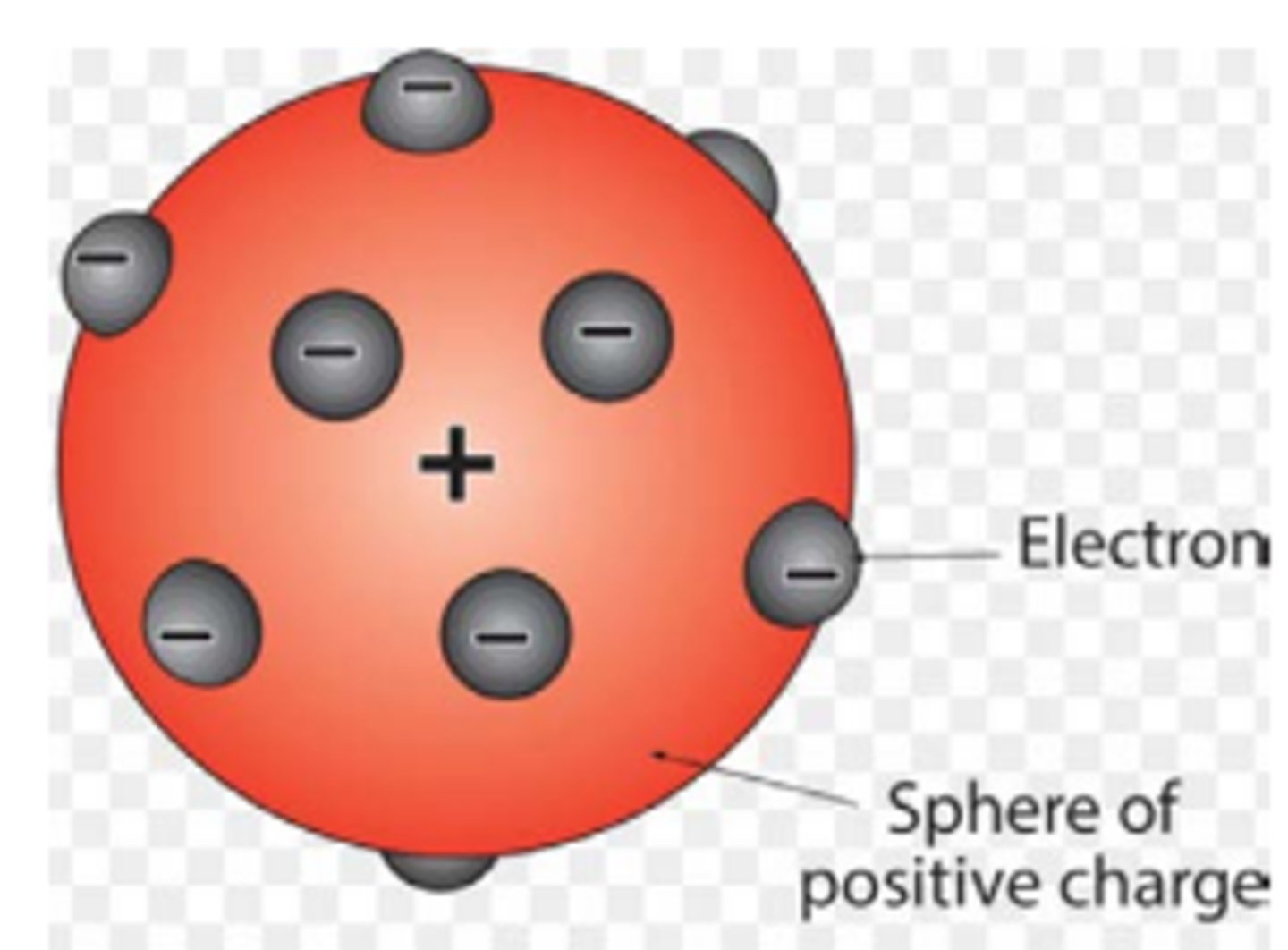
What is meant by the plum pudding atom model? (1)
This is a neutral atom which is a sphere of positive charge with tiny electrons embedded in it.
What was the purpose of Rutherford's alpha scattering experiment? (1)
To test and ultimately disprove the Plum Pudding model.
How was Rutherford's alpha scattering experiment carried out? (3)
- Alpha particles were fired at a very thin sheet of gold foil in a vacuum.
- The deflected alpha particles were detected by scintillators (material that released photons when hit).
- These observations changed the model of the atom.
What were the observations from Rutherford's alpha scattering experiment? (3)
- Most alpha particles passed through the foil without any deflection.
- Some alpha particles were deflected by small angles.
- Very few alpha particles were reflected by the foil and travelled back.
What did the observations provide evidence for from Rutherford's alpha scattering experiment? (3)
- The atom is mostly empty space as most alpha particles travelled straight through.
- The nucleus is positively charged as some alpha particles were deflected.
- The nucleus contained most of the atoms mass as sometimes an alpha particle was reflected backwards.
What is Rutherford's atomic model? (2)
- This is a mostly empty atom with a small dense nucleus containing protons and neutrons.
- The nucleus is surrounded by orbiting electrons.
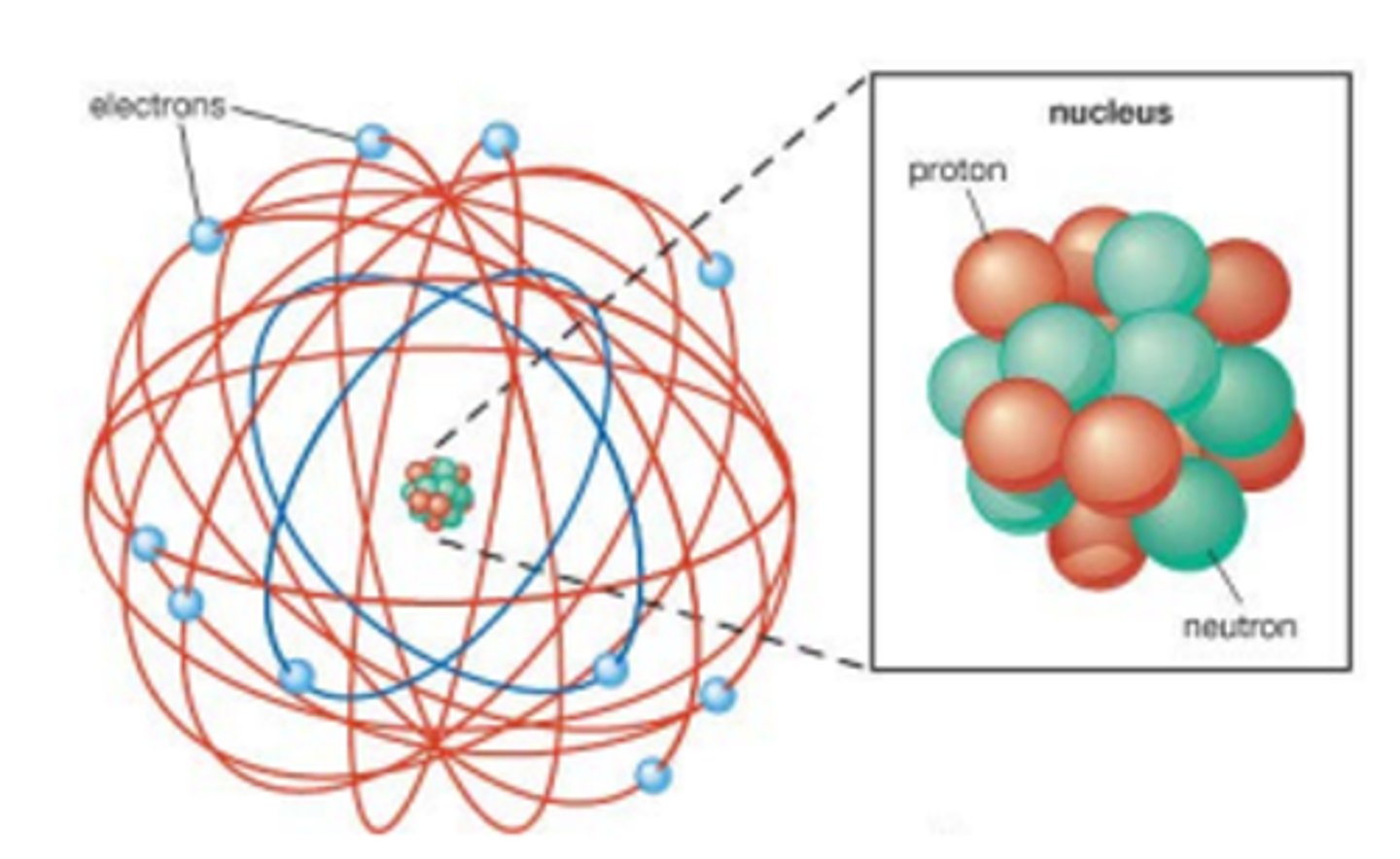
What is the nucleus made out of? (1)
The nucleus is made out of protons and neutrons.
What orbits the nucleus? (1)
Electrons orbit the nucleus in Rutherford's model.
What is the charge of a proton? (1)
This is equal to the fundamental charge (e), 1.6 × 10⁻¹⁹ C
What is the charge of an electron? (1)
This is equal to the negative of the fundamental charge (-e), 1.6 × 10⁻¹⁹ C
How is an atom kept neutral? (1)
As there are the same amount of protons as electrons, the positive charge cancels out the negative charge and the atom is neutral.
How much larger is the atom compared to the nucleus? (1)
The diameter of the nucleus (at 10⁻¹⁵ m) is 100,000 times smaller than the diameter of the atom (at 10⁻¹⁰ m)
What is meant by the proton number? (1)
This is the amount of protons in an atom.
What is meant by the nucleon number? (1)
This is the amount of nucleons (protons + neutrons) in an atom.
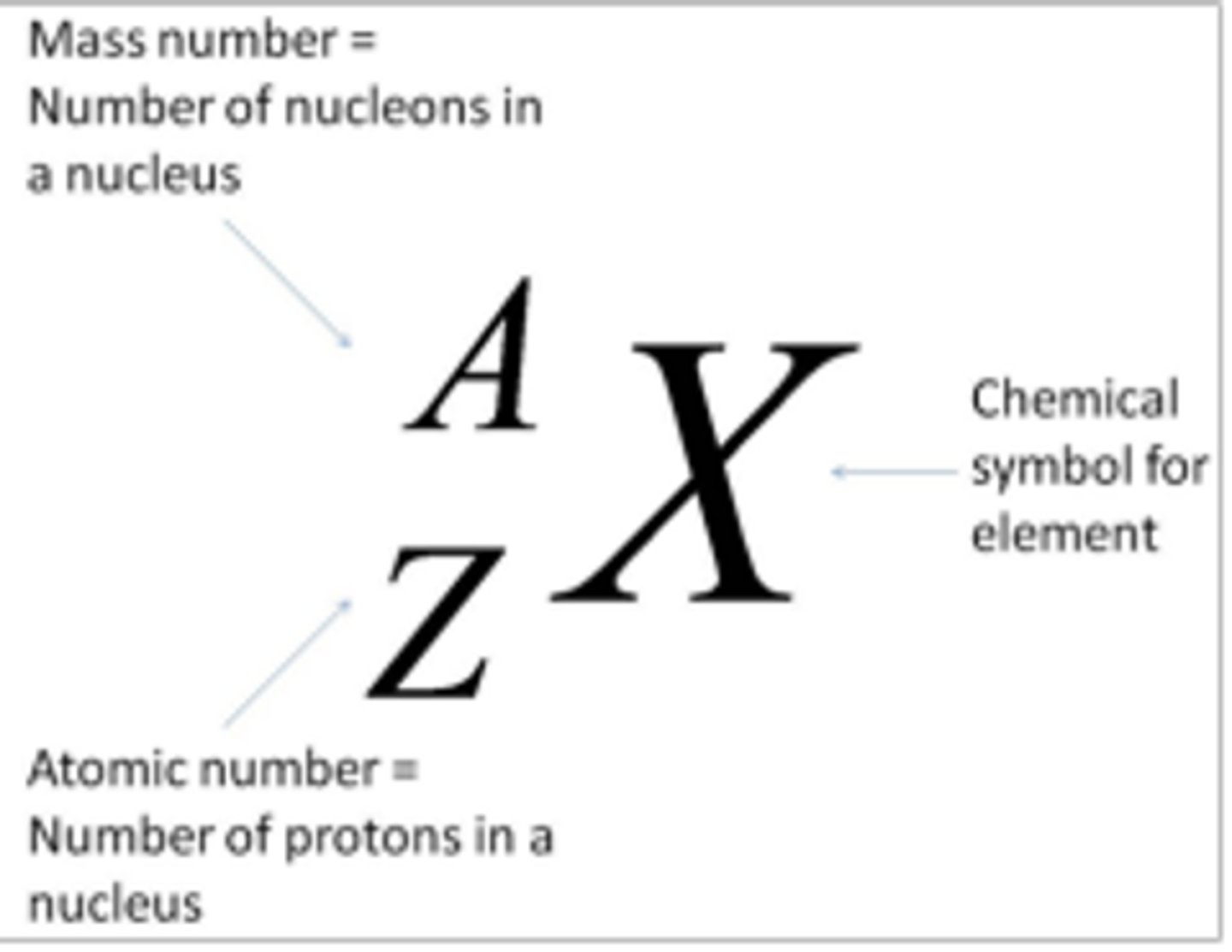
How are atoms represented in terms of their proton and nucleon numbers? (1)
What is meant by an isotope? (2)
- These are atoms of the same element with the same amount of protons but differing amount of neutrons.
- They will undergo different nuclear reactions due to their different stability.
What is a femtometre (fm) (1)
1 fm is equal to 10⁻¹⁵ m
What is the strong nuclear force? (3)
- This is one of the four fundamental forces (gravity, strong nuclear force, weak nuclear force and electromagnetism).
- This acts between all hadrons (quarks and nucleons) and counteracts the repulsive electrostatic forces between protons in the atom.
- It is attractive at small distances (up to 3 fm) and repulsive at very small distances (below 0.5 fm) and has a very small range.
What are the conditions for the strong nuclear force to be attractive or repulsive? (1)
It is attractive at small distances (up to 3 fm) and repulsive at very small distances (below 0.5 fm) and has a very small range.
What is the equation for the radius of a nuclei? (2)
- This is given by: R = r₀A^(1/3)
- Where r_0 is a constant spacing of around 1.2 fm and A is the nucleon number of the nuclei.
What is meant by the nuclear density? (2)
- This is the density of the nucleus of the atom.
- It is the mass of the nucleus divided by the volume of the atom.
What is an antiparticle? (1)
Every particle has a corresponding antiparticle which has an equal mass but an opposite charge and are attracted to each other causing them to annihilate.
What is meant by annihilation? (1)
This is when a particle and an antiparticle meet at a point in space and annihilate to produce energy in the form of photons.
What is the antiparticle of the electron? (1)
This is the positron (as it has a positive charge).
What is the antiparticle of the proton? (1)
This is called the antiproton (as it has opposite charge).
What is the antiparticle of the neutron? (1)
This is called the anti-neutron.
What is the antiparticle of the neutrino? (1)
This is called the antineutrino.
What is a hadron? (1)
These are particles which are made out of quarks and are acted on by the strong and weak nuclear forces.
What are some examples of hadrons? (1)
Protons and neutrons are both hadrons (baryons).
What is a meson? (1)
These are particles that exist in particle-antiparticle pairs such as the pion.
What is a baryon? (1)
These are particles made out of 3 quarks such as a proton and neutron.
What is a lepton? (1)
This is a type of fundamental particle which is not affected by the strong force but are affected by the weak nuclear force.
What are some examples of leptons? (1)
The electron and neutrino (ν) are both examples of leptons.
What are the different types of quarks and their charges? (3)
These are the quarks needed in the OCR specification:
- The up quark (u) which has a charge of (2/3) e
- The down quark (d) which has a charge of (-1/3) e
- The strange quark (s) which has a charge of (+2/3) e
What are the different types of antiquarks and their charges? (3)
These are the antiquarks needed in the OCR specification:
- The anti-up quark (u} which has a charge of (-2/3) e
- The anti-down quark (d) which has a charge of (+1/3) e
- The anti-strange quark (s) which has a charge of (-2/3) e
What quarks is a proton made out of? (1)
Protons are made out of 2 up quarks and 1 down quark resulting in a charge of +e
What quarks is a neutron made out of? (1)
A neutron is made out of 1 up quark and 2 down quarks to keep a neutral charge of zero.
What is meant by beta-minus decay? (2)
- This is where the weak nuclear force changes a down quark into an up quark within a neutron turning it into a proton.
- This releases a high speed electron (beta-minus particle) and an antineutrino to conserve charge.
What is the equation for beta-minus decay? (1)
This is given by:
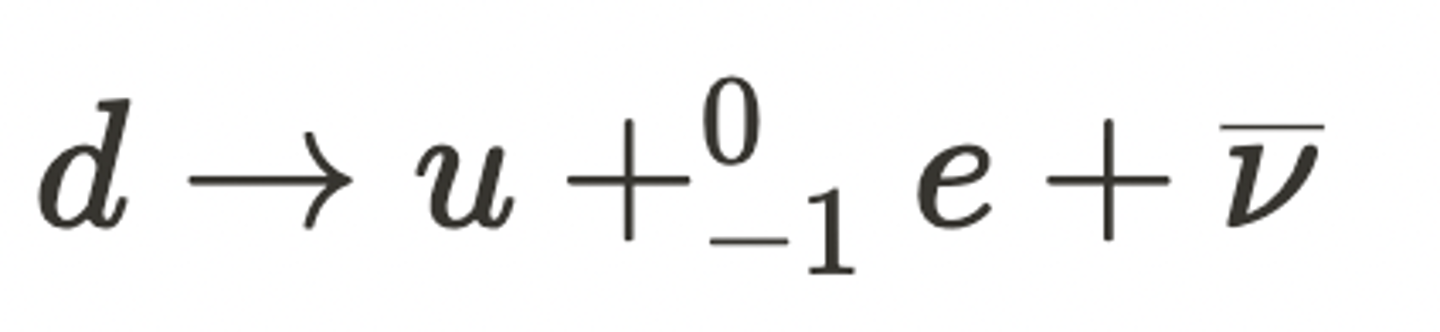
What is meant by beta-plus decay? (2)
- This is where the weak nuclear force changes an up quark into a down quark within a proton turning it into a neutron.
- This releases a high speed antielectron (beta-plus particle) and an neutrino to conserve charge.
What is the equation for beta-plus decay? (1)
This is given by:
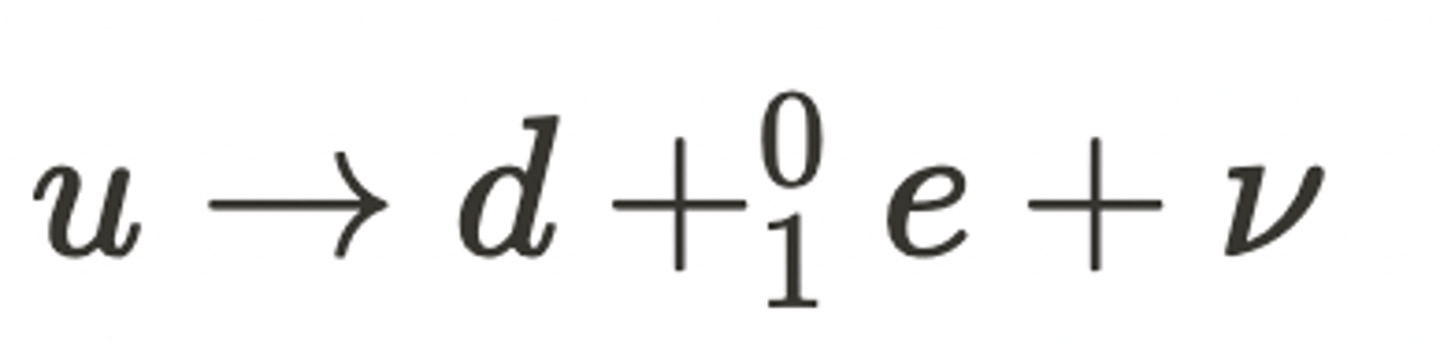
How are quark transformation equations balanced? (2)

How do particles decay in the quark model? (1)
Particles can decay to form other particles as long as the charge is conserved.
What is meant by radioactive decay? (2)
- This is the decay of a nucleus resulting in the release of energy and matter from the nucleus.
- It is a spontaneous and random process.
What is meant by a spontaneous process? (1)
This is a process that occurs without any external input to the system.
What is meant by a random process? (1)
This is where it is impossible to predict the next thing to happen in a process (e.g impossible to predict the next atomic nucleus to decay.)
What is the pattern of radioactive decay? (2)
- If there is a large enough number of nuclei the decay becomes predictable.
- The decay follows the half life which is the amount of time for the sample to halve.
What is meant by ionising power? (1)
This means radiation that has enough energy to break an electron off an atom. High ionising power means it usually can ionise atoms.
What is an alpha particle? (1)
This is a form of radiation which is made out of a helium nucleus.
What are the properties of alpha radiation? (3)
- It travels slowly.
- High ionising ability.
- Low penetrating ability and can be stopped by paper.
What is a beta particle? (1)
This is a form of radiation which is a fast-travelling electron.
What are the properties of beta radiation? (3)
- It travels quickly.
- It has a medium ionising power.
- It has a medium penetrating paper and can be stopped by aluminium.
What is a gamma ray? (1)
This is a form of radiation which is an electromagnetic wave in the gamma part of the spectrum.
What are the properties of gamma radiation? (3)
- It travels at the speed of light.
- It has zero ionising power.
- It has very high penetrating power and can be stopped by thick layers of lead.
How can the different types of radiation be investigated? (2)
- Put a source of radiation in front of different materials such as paper, aluminium and lead.
- The levels of activity can be measured and used to see which type of radiation is being emitted due to the differing penetration power.
What are some examples of radiation detectors? (2)
- A bubble chamber which forms tracks of bubbles when ionising radiation passes through.
- A Geiger-Muller tube which forms cascades of electrons when an ionising particle hits the absorber.
What is alpha decay? (2)
- This is the loss of an alpha particle (helium nucleus) from an unstable nuclei.
- The binding energy increases but the energy of the nucleus will decrease.
What is the equation for alpha decay? (3)
- This is written as:
- This is where the nuclei X decays into Y with the emission of an alpha particle.
- The nucleon and proton number decreases accordingly.
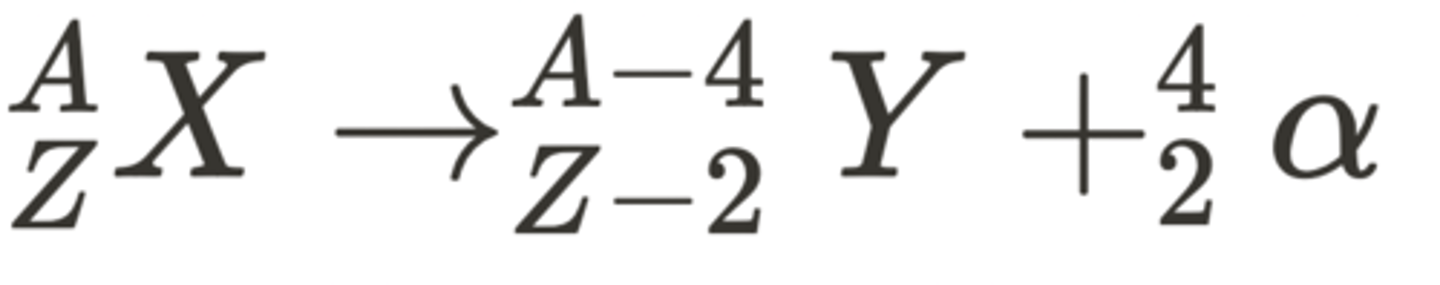
How are nuclear transformation equations balanced? (1)
The nucleon number and proton number must be kept constant on both sides of the equation.
What is meant by the activity of a source? (1)
This is the rate at which nuclei decay and can also be defined as the number of decays per second.
What is the activity of a source measured in? (1)
This is measured in Becquerel in units of s⁻¹
What factors affect the activity of a source? (2)
- The half-life of the source.
- The number of the active nuclei (N).
How does the half-life affect the activity of a source? (1)
If the half-life is short, the number of unstable nuclei will decrease by a lot and the activity will be decreased.
How does the number of active nuclei affect the activity of a source? (1)
If there is less active nuclei, there will be less decays and a lower activity.
What is the decay constant (λ)? (1)
This is the probability that an individual nucleus will decay per unit time. It is measured in s⁻¹
What is the equation for the activity of a source? (2)
- As the activity is directly proportional to the number of active nuclei with the decay constant as the constant of proportionality this is given by: A = λN
- Where A is the activity (Bq), λ is the decay constant (s⁻¹) and N is the number of active nuclei.
What is the equation for exponential decay of the number of undecayed nuclei? (2)
- This is given by: N = N₀e^-λt
- Where N is the number of undecayed nuclei, N₀ is the initial number of nuclei when t = 0, λ is the decay constant (s⁻¹) and t is time (s).
What is the equation for exponential decay of the activity of nuclei? (2)
- As A = λN the equation N = N₀e^-λt becomes: A = A₀e^λt
- Where A is the activity of the nuclei (Bq), A₀ is the initial activity of the nuclei when t = 0, λ is the decay constant (s⁻¹) and t is the time (s).
How can radioactive decay be modelled using dice? (2)
- If a large sample of dice is rolled, (and landing on a 6 represents a decay and must be taken out of the sample) radioactive decay can be modelled.
- It is completely random to get a 6 and have a decay but the chance of getting a 6 as the number of dice decrease just like radioactive decay.
What is meant by the half-life of a decay (t₁/₂)? (1)
This is the time taken for half a sample of unstable nuclei to decay or the time taken for the activity of the nuclei to halve.
What is the equation for the half-life? (2)
- If N = N₀/2 is substituted into the equation N = N₀ e^λt and rearranged for time (half-life):
- t₁/₂ = ln 2
How can the equation for radioactive decay be modelled using graphical methods? (3)
- The equation ∆N/∆t = -λN can be modelled using a graph.
- The number of nuclei can be measured at regular time intervals and a graph of number of nuclei against time can be plotted.
- The gradient should be equal to - λN
How can the equation for radioactive decay be modelled using a spreadsheet? (3)
- The equation ∆N/∆t = -λN can be modelled using a spreadsheet.
- A table of number of nuclei at regular time intervals can be created.
- The pattern should follow the above equation.
What is meant by radioactive dating? (1)
This is where absorbed radiation in materials is analysed to find the age of an object.
What is meant by carbon dating? (3)
- This is done by measuring the ratio of the Carbon-14 isotope to the Carbon-12 isotope to find the age of dead tissue.
- This works as C-14 atoms are absorbed by organisms and the ratio of C-14 to C-12 will match the atmosphere and C-14 decays with a half life of 5400 years.
- Comparing the atmospheric ratio to the ratio in the dead tissue can estimate when an organism died.
What is meant by mass-energy equivalence? (1)
Mass and energy are manifestations of the same thing and mass can be thought of as energy in the form mass-energy.
What is Einstein's mass-energy equation? (2)
- This is given by ΔE = Δmc²
- Where ∆E is the change in energy (J), ∆m is the change in mass (kg) and c is the speed of light (3 × 10⁸ m/s⁻¹)
How is energy released (or absorbed) in nuclear reactions? (2)
- When radiation is emitted from nuclei there is a reduction in mass (e.g alpha particle being emitted) which is converted to energy through ΔE = Δmc²
- Energy can be absorbed to which means the mass must increase.
What is meant by the mass defect? (2)
- This is the difference of mass between the products and the reactants of a nuclear reaction.
- It can also be defined as the difference of the mass between the constituent atoms of the nucleus and the nucleus.
What causes the mass defect? (1)
The mass loss is due to the potential of the strong and electrostatic forces, it can also be seen as work required to separate constituent nucleons.
What is meant by the binding energy? (1)
This is defined as the minimum energy required to break a nucleus into its constituent components.
What is meant by the binding energy per nucleon? (1)
This is the total binding energy to break the entire nucleus split to find the binding energy per nucleon, this makes it easier to compare elements in terms of their binding energy.
What does the binding energy per nucleon against nucleon number graph look like? (1)
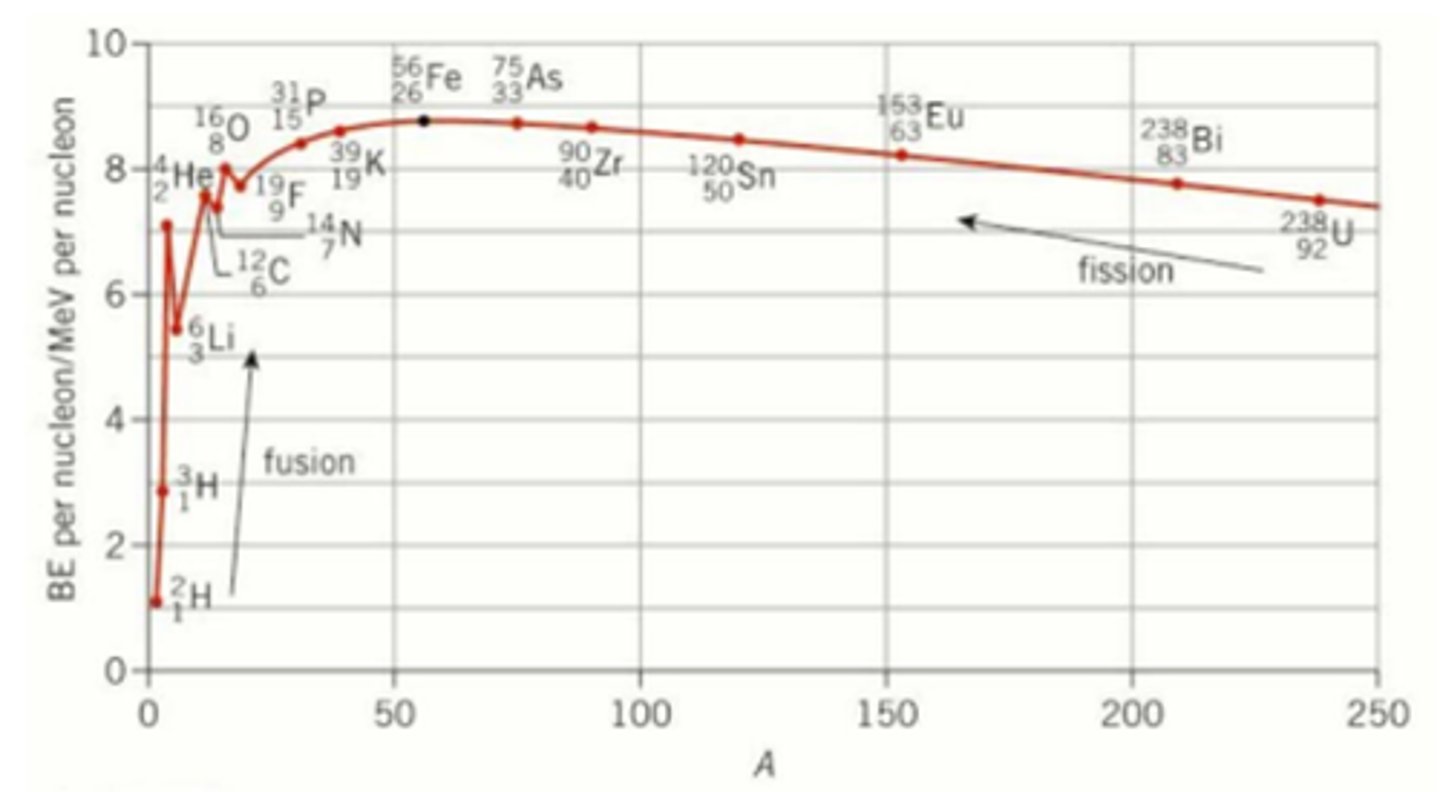
What is the most stable element? (1)
This is iron as it has the most binding energy per nucleon.
How do elements react with a nucleon number below 56? (3)
- Atoms will fuse together to make higher nucleon number elements.
- This makes the binding energy per nucleon increase due to the increase in the strong force attraction compared to the small increase in electrostatic repulsion.
- This will release energy due to the mass defect.
How do elements react with a nucleon number above 56? (3)
- When elements react the nuclei will break apart and make lower nucleon number elements by emitting radiation.
- This is because of the strong electrostatic repulsion and a weaker strong force interaction.
- The binding energy per nucleon will decrease and energy will be released due to a fission reaction.
How does the binding energy relate to the mass-energy equation? (1)
The binding energy of the nucleus can be found by using the equation ΔE = Δmc²
What is meant by nuclear fission? (1)
This is the breaking apart of larger nuclei into smaller nuclei causing a reduction in the total binding energy, a mass defect and a resulting emission of energy.
How is an induced nuclear fission reaction carried out? (3)
- A neutron is fired at Uranium-235 (as it easily undergoes fission and is abundant) and it becomes Uranium-236 which is very unstable.
- The isotope then decays via fission splitting into 2 daughter nuclei.
- There is a mass defect which is converted into energy.
Which element is commonly used in nuclear fission? (1)
Uranium-235 as it easily undergoes fission and is abundant.
What is meant by a chain reaction in nuclear fission? (2)
- The daughter nuclei released from Uranium-236 could also be unstable and decay, further releasing energy.
- This process can repeat again and again releasing a lot of energy. This process is used in fission reactions.
What is the structure of a fission reactor?
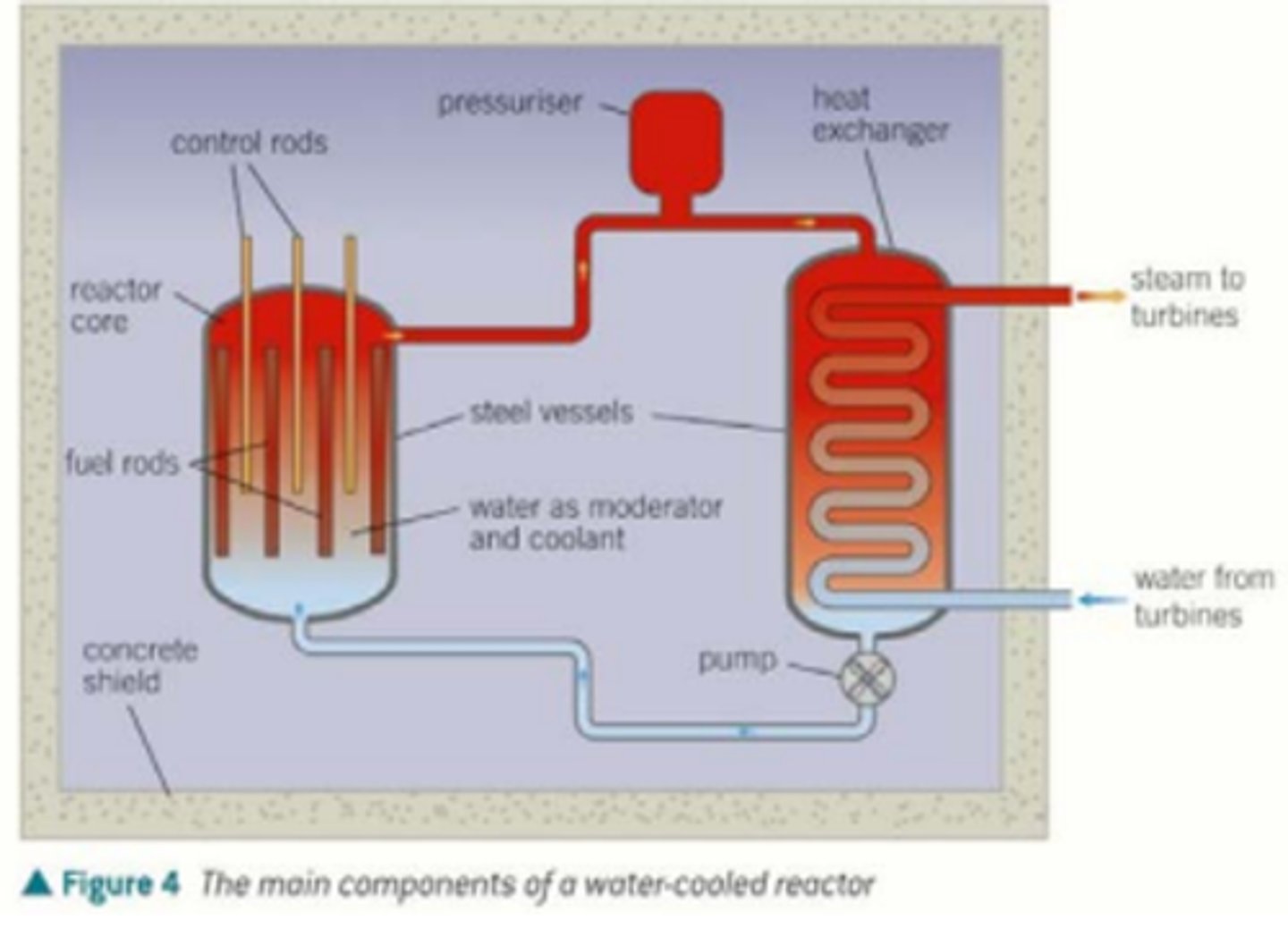
What is the reactor core? (1)
This is the core of a fission reactor where the nuclear fission takes place.
What are fuel rods in a fission reactor? (1)
This is in the reactor core and contains the material that will undergo nuclear fission.
What are control rods in a fission reactor? (1)
Control rods absorb neutrons from reactions to prevent further nuclear fission and prevent a huge chain reaction.
What is a moderator in a fission reactor? (1)
Moderators (e.g water and graphite) slow the speed of neutrons and increases the chance of fission reactions.
What is the environmental impact of nuclear waste? (2)
- The final products of nuclear fission are radioactive and are toxic waste.
- It is usually buried deep underground and in lead boxes so radiation cannot leak out.
What is meant by nuclear fusion? (1)
This is where smaller nuclei are fused together to form larger nuclei. This produces a mass defect and releases energy.
What are the conditions for nuclear fusion to occur? (2)
- There must be high temperatures so the nuclei have enough kinetic energy to overcome electrostatic repulsion between them and fuse together.
- There must be high pressures so the nuclei are close enough so they can fuse.
What are the different types of nuclear fusion processes? (3)
- In stars like the sun, a proton-proton chain takes place where lone protons fuse to form an unstable helium nucleus which decays into deuterium nuclei. This then fuses with another proton and this continues until a stable helium nucleus is formed.
- In larger stars the CNO cycle occurs where carbon, nitrogen and oxygen fuse to form larger elements.
- Artificial fusion can occur on earth but high temperatures and pressures required means they can only run for a short period of time.