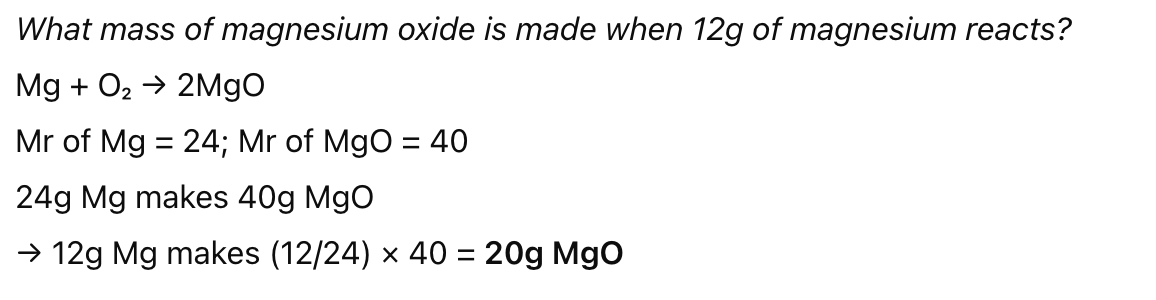Chemistry IGCSE - Stoichiometry
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Molecular formula of a compound
the number and type of atoms in one molecule
Relative atomic mass (Ar)
average mass of the isotopes of an element compared to the 1/12th of the mass of 12C
Relative molecular mass (Mr)
the sum of the relative atomic masses. (ONLY FOR IONIC COMPOUNDS)
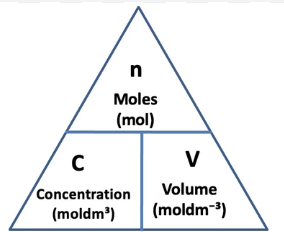
Concentration UOM
g/dm3
Mole (UOM: mol)
Avogadro’s constant → 6.02 × 1023 particles
unit of amount of substance
Mole equation
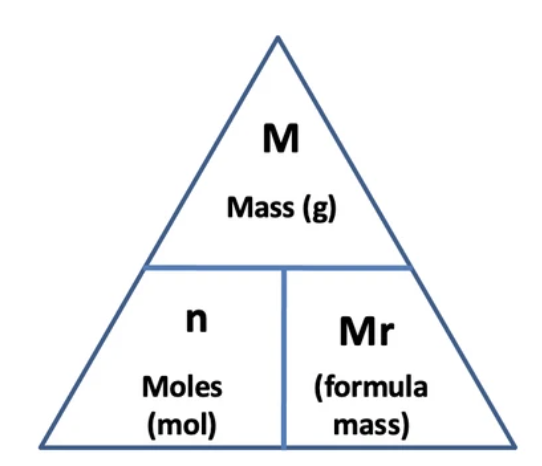
Conversion between cm3 to dm3
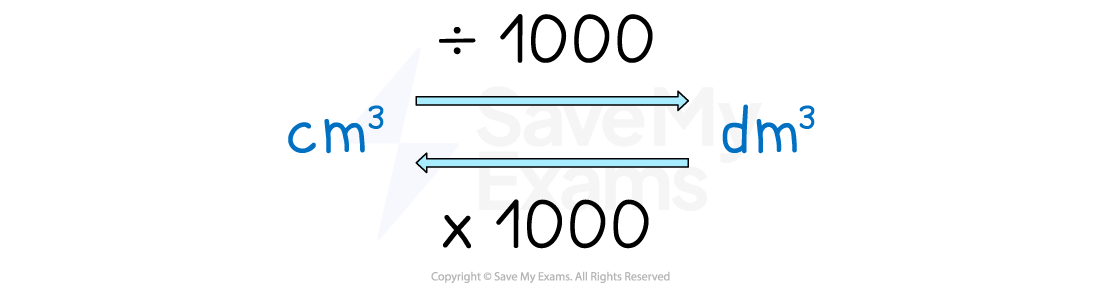
Concentration formula
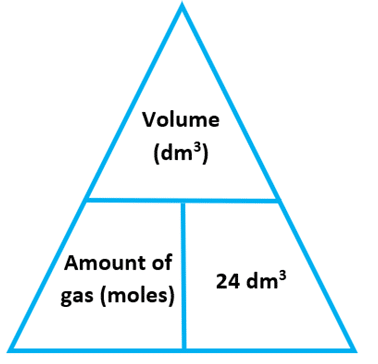
Molar gas volume formula
Calculating reacting masses in small proportions (not using mole)
Write balanced equation
Use given mass + relative formula mass → find how many ‘parts’ of a substance are reacting
Use ratio from equation to find unknown mass